Thermoelectric, Electrochemical, & Dielectric Properties of Four ZnO Nanostructures
- PMID: 36556622
- PMCID: PMC9784509
- DOI: 10.3390/ma15248816
Thermoelectric, Electrochemical, & Dielectric Properties of Four ZnO Nanostructures
Abstract
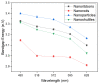
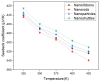
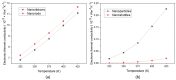
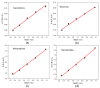
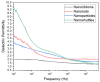
In this work, we investigated the thermoelectric, electrochemical, and dielectric properties of four different ZnO morphologies, namely nanoribbons, nanorods, nanoparticles, and nanoshuttles. Temperature-dependent Seebeck coefficients were observed using thermoelectric measurements, which confirmed that all synthesized ZnO nanostructures are n-type semiconductors. The Van der Pauw method was applied to measure electrical conductivity, which was also used to calculate the thermal activation energy. Electrochemical properties were analyzed by cyclic voltammetry techniques under five different optical filters. Electrical conductivity of ZnO morphologies showed an increasing trend with increasing temperature. The highest electrical conductivity (1097.60 Ω−1 m−1) and electronic thermal conductivity (1.16×10−4 W/mK) were obtained for ZnO nanorods at 425 K, whereas ZnO nanoshuttles carried the lowest electrical conductivity (1.10 × 10−4 Ω−1 m−1) and electronic thermal conductivity (8.72 × 10−7 W/mK) at 325 K. ZnO nanorods obtained the maximum Power factor value in all temperature ranges. All nanostructures showed electro-catalytic performance with different optical filters. From impedance spectroscopy analysis, ZnO nanorods showed the highest dielectric constant at high frequencies (>1 MHz) at 2.02 ± 0.06, while ZnO nanoshuttles gave the highest dielectric constant at low frequencies (<100 Hz) at 9.69 ± 0.05. These results indicate that ZnO nanorods have the most favorable thermoelectric, electrochemical, and dielectric properties compared to all other ZnO morphologies.
Keywords: Seebeck coefficient; cyclic voltammetry; dielectric constant; electrochemical; impedance spectroscopy; thermal conductivity; thermoelectric.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Comparative investigations of structural, electronic, optical, and thermoelectric properties of pure and 2 at. % Al-doped ZnO.J Mol Model. 2023 Oct 17;29(11):343. doi: 10.1007/s00894-023-05750-x. J Mol Model. 2023. PMID: 37847327
-
ZnO@TiO2 Core Shell Nanorod Arrays with Tailored Structural, Electrical, and Optical Properties for Photovoltaic Application.Molecules. 2019 Nov 1;24(21):3965. doi: 10.3390/molecules24213965. Molecules. 2019. PMID: 31683868 Free PMC article.
-
A Study on the Relationship between the Thermoelectric Properties and Infrared Emission Performance of ZnO/SrTiO3 Composite Materials.ACS Appl Mater Interfaces. 2024 Oct 2;16(39):52712-52718. doi: 10.1021/acsami.4c13105. Epub 2024 Sep 19. ACS Appl Mater Interfaces. 2024. PMID: 39295293
-
Direct Probing of Cross-Plane Thermal Properties of Atomic Layer Deposition Al2O3/ZnO Superlattice Films with an Improved Figure of Merit and Their Cross-Plane Thermoelectric Generating Performance.ACS Appl Mater Interfaces. 2018 Dec 26;10(51):44472-44482. doi: 10.1021/acsami.8b15997. Epub 2018 Dec 13. ACS Appl Mater Interfaces. 2018. PMID: 30507128
-
Thermoelectric Transport Properties of Fe-Enriched ZnO with High-Temperature Nanostructure Refinement.ACS Appl Mater Interfaces. 2015 Apr 22;7(15):7927-37. doi: 10.1021/am509050a. Epub 2015 Apr 10. ACS Appl Mater Interfaces. 2015. PMID: 25839985
Cited by
-
Advancing Thermoelectric Materials: A Comprehensive Review Exploring the Significance of One-Dimensional Nano Structuring.Nanomaterials (Basel). 2023 Jul 5;13(13):2011. doi: 10.3390/nano13132011. Nanomaterials (Basel). 2023. PMID: 37446526 Free PMC article. Review.
-
A semi-transparent thermoelectric glazing nanogenerator with aluminium doped zinc oxide and copper iodide thin films.Commun Eng. 2024 Oct 15;3(1):145. doi: 10.1038/s44172-024-00291-4. Commun Eng. 2024. PMID: 39407012 Free PMC article.
References
-
- Zhi-gang J., Kuan-Kuan P., Yan-Hua L., Rong-sun Z. Preparation and photocatalytic performance of porous ZnO microrods loaded with Ag. Trans. Nonferrous Met. Soc. China. 2012;22:873–878.
-
- Thangeeswari T., George A.T., Kumar A.A. Optical Properties and FTIR Studies of Cobalt Doped ZnO Nanoparticles by Simple Solution Method. Indian J. Sci. Technol. 2016;9:1–4. doi: 10.17485/ijst/2016/v9i1/85776. - DOI
-
- Wang Z.L. Nanostructures of zinc oxide. Mater. Today. 2004;7:26–33.
-
- Topoglidis E., Cass A.E.G., O’Regan B., Durrant J.R. Immobilisation and bioelectrochemistry of proteins on nanoporous TiO2 and ZnO films. J. Electroanal. Chem. 2001;517:20–27. doi: 10.1016/S0022-0728(01)00673-8. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

