Large Animal Models for Simulating Physiology of Transfusion of Red Cell Concentrates-A Scoping Review of The Literature
- PMID: 36556937
- PMCID: PMC9787038
- DOI: 10.3390/medicina58121735
Large Animal Models for Simulating Physiology of Transfusion of Red Cell Concentrates-A Scoping Review of The Literature
Abstract
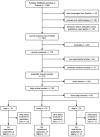
Background and Objectives: Transfusion of red cell concentrates is a key component of medical therapy. To investigate the complex transfusion-associated biochemical and physiological processes as well as potential risks for human recipients, animal models are of particular importance. This scoping review summarizes existing large animal transfusion models for their ability to model the physiology associated with the storage of erythrocyte concentrates. Materials and Methods: The electronic databases PubMed, EMBASE, and Web of Science were systematically searched for original studies providing information on the intravenous application of erythrocyte concentrates in porcine, ovine, and canine animal models. Results: A total of 36 studies were included in the analysis. The majority of porcine studies evaluated hemorrhagic shock conditions. Pig models showed high physiological similarities with regard to red cell physiology during early storage. Ovine and canine studies were found to model typical aspects of human red cell storage at 42 days. Only four studies provided data on 24 h in vivo survival of red cells. Conclusions: While ovine and canine models can mimic typical human erythrocyte storage for up to 42 days, porcine models stand out for reliably simulating double-hit pathologies such as hemorrhagic shock. Large animal models remain an important area of translational research since they have an impact on testing new pharmacological or biophysical interventions to attenuate storage-related adverse effects and allow, in a controlled environment, to study background and interventions in dynamic and severe disease conditions.
Keywords: blood transfusion; erythrocyte storage; large animal model; red cell concentrates; transfusion model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Ovine red cell concentrates for transfusion research - is the storage lesion comparable to human red cell concentrates?Vox Sang. 2021 May;116(5):524-532. doi: 10.1111/vox.13020. Epub 2020 Oct 26. Vox Sang. 2021. PMID: 33107065
-
[The importance of quality of whole blood and erythrocyte concentrates for autologous transfusion. A literature survey and meta-analysis of in vivo erythrocyte recovery].Anaesthesist. 1996 Aug;45(8):694-707. doi: 10.1007/s001010050302. Anaesthesist. 1996. PMID: 8967582 German.
-
The red cell storage lesion(s): of dogs and men.Blood Transfus. 2017 Mar;15(2):107-111. doi: 10.2450/2017.0306-16. Blood Transfus. 2017. PMID: 28263166 Free PMC article. Review.
-
In vitro and in vivo validation of stored swine erythrocyte viability to establish an experimental model of homologous red blood cell transfusion: a pilot study.Rev Bras Ter Intensiva. 2014 Jul-Sep;26(3):287-91. doi: 10.5935/0103-507x.20140040. Rev Bras Ter Intensiva. 2014. PMID: 25295823 Free PMC article.
-
Perioperative Red Blood Cell Transfusion: What We Do Not Know.Chin Med J (Engl). 2015 Sep 5;128(17):2383-6. doi: 10.4103/0366-6999.163384. Chin Med J (Engl). 2015. PMID: 26315088 Free PMC article. Review.
Cited by
-
Preterm Piglets Born by Cesarean Section as a Suitable Animal Model for the Study of Iron Metabolism in Premature Infants.Int J Mol Sci. 2024 Oct 18;25(20):11215. doi: 10.3390/ijms252011215. Int J Mol Sci. 2024. PMID: 39456997 Free PMC article.
-
Breaking barriers in trauma research: A narrative review of opportunities to leverage veterinary trauma for accelerated translation to clinical solutions for pets and people.J Clin Transl Sci. 2024 Apr 5;8(1):e74. doi: 10.1017/cts.2024.513. eCollection 2024. J Clin Transl Sci. 2024. PMID: 38715566 Free PMC article. Review.
References
-
- Donadee C., Raat N.J., Kanias T., Tejero J., Lee J.S., Kelley E.E., Zhao X., Liu C., Reynolds H., Azarov I., et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources