A Reduction of Calcineurin Inhibitors May Improve Survival in Patients with De Novo Colorectal Cancer after Liver Transplantation
- PMID: 36556957
- PMCID: PMC9785597
- DOI: 10.3390/medicina58121755
A Reduction of Calcineurin Inhibitors May Improve Survival in Patients with De Novo Colorectal Cancer after Liver Transplantation
Abstract
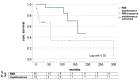
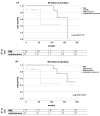
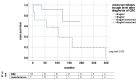
Background and Objectives: After liver transplantation (LT), long-term immunosuppression (IS) is essential. IS is associated with de novo malignancies, and the incidence of colorectal cancer (CRC) is increased in LT patients. We assessed course of disease in patients with de novo CRC after LT with focus of IS and impact on survival in a retrospective, single-center study. Materials and Methods: All patients diagnosed with CRC after LT between 1988 and 2019 were included. The management of IS regimen following diagnosis and the oncological treatment approach were analyzed: Kaplan−Meier analysis as well as univariate and multivariate analysis were performed. Results: A total of 33 out of 2744 patients were diagnosed with CRC after LT. Two groups were identified: patients with restrictive IS management undergoing dose reduction (RIM group, n = 20) and those with unaltered regimen (maintenance group, n = 13). The groups did not differ in clinical and oncological characteristics. Statistically significant improved survival was found in Kaplan−Meier analysis for patients in the RIM group with 83.46 (8.4−193.1) months in RIM and 24.8 (0.5−298.9) months in the maintenance group (log rank = 0.02) and showed a trend in multivariate cox regression (p = 0.054, HR = 14.3, CI = 0.96−213.67). Conclusions: Immunosuppressive therapy should be reduced further in patients suffering from CRC after LT in an individualized manner to enable optimal oncological therapy and enable improved survival.
Keywords: colorectal carcinoma; de novo malignancy; immunosuppression; liver transplantation.
Conflict of interest statement
All authors declare no conflicts of interest related to the presented work.
Figures


Similar articles
-
Immunosuppressive Treatment With mTOR Inhibitors for Malignancies After Liver Transplantation: Long-Term Survival Retrospective Analysis.Transplant Proc. 2020 Jun;52(5):1507-1510. doi: 10.1016/j.transproceed.2020.02.058. Epub 2020 Mar 22. Transplant Proc. 2020. PMID: 32213292
-
Calcineurin-Inhibitor Discontinuation Could Reduce the Risk of De Novo Malignancies After Liver Transplantation for Alcohol-Related Liver Disease.Clin Transplant. 2024 Oct;38(11):e70014. doi: 10.1111/ctr.70014. Clin Transplant. 2024. PMID: 39552184
-
Conversion from Calcineurin Inhibitor-Based Immunosuppression to Mycophenolate Mofetil in Monotherapy Reduces Risk of De Novo Malignancies After Liver Transplantation.Ann Transplant. 2017 Mar 17;22:141-147. doi: 10.12659/aot.901556. Ann Transplant. 2017. PMID: 28302995
-
'De novo' and 'recurrent' autoimmune hepatitis after liver transplantation: A comprehensive review.J Autoimmun. 2016 Jan;66:17-24. doi: 10.1016/j.jaut.2015.08.017. Epub 2015 Sep 14. J Autoimmun. 2016. PMID: 26377632 Review.
-
Modification of immunosuppressive therapy as risk factor for complications after liver transplantation.Best Pract Res Clin Gastroenterol. 2017 Apr;31(2):199-209. doi: 10.1016/j.bpg.2017.03.001. Epub 2017 Apr 12. Best Pract Res Clin Gastroenterol. 2017. PMID: 28624108 Review.
Cited by
-
Dynamics of torque teno virus load in kidney transplant recipients with indication biopsy and therapeutic modifications of immunosuppression.Front Med (Lausanne). 2024 Jan 24;11:1337367. doi: 10.3389/fmed.2024.1337367. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38327708 Free PMC article.
-
Therapeutic Landscapes in Colorectal Carcinoma.Medicina (Kaunas). 2023 Apr 23;59(5):821. doi: 10.3390/medicina59050821. Medicina (Kaunas). 2023. PMID: 37241053 Free PMC article.
References
-
- Geissler E.K., Schnitzbauer A.A., Zülke C., Lamby P.E., Proneth A., Duvoux C., Burra P., Jauch K.W., Rentsch M., Ganten T.M., et al. Sirolimus Use in Liver Transplant Recipients With Hepatocellular Carcinoma: A Randomized, Multicenter, Open-Label Phase 3 Trial. Transplantation. 2016;100:116–125. doi: 10.1097/TP.0000000000000965. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

