Recent Progress in Silicon Carbide-Based Membranes for Gas Separation
- PMID: 36557162
- PMCID: PMC9783330
- DOI: 10.3390/membranes12121255
Recent Progress in Silicon Carbide-Based Membranes for Gas Separation
Abstract
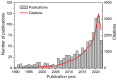
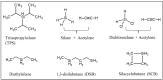
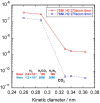
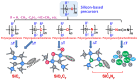
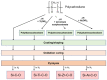
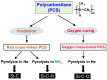
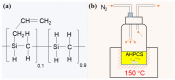
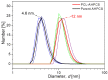
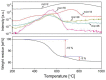
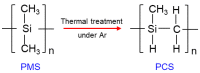
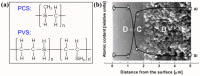
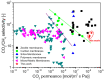
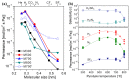
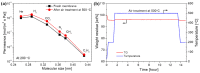
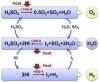
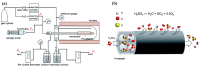
The scale of research for developing and applying silicon carbide (SiC) membranes for gas separation has rapidly expanded over the last few decades. Given its importance, this review summarizes the progress on SiC membranes for gas separation by focusing on SiC membrane preparation approaches and their application. The precursor-derived ceramic approaches for preparing SiC membranes include chemical vapor deposition (CVD)/chemical vapor infiltration (CVI) deposition and pyrolysis of polymeric precursor. Generally, SiC membranes formed using the CVD/CVI deposition route have dense structures, making such membranes suitable for small-molecule gas separation. On the contrary, pyrolysis of a polymeric precursor is the most common and promising route for preparing SiC membranes, which includes the steps of precursor selection, coating/shaping, curing for cross-linking, and pyrolysis. Among these steps, the precursor, curing method, and pyrolysis temperature significantly impact the final microstructures and separation performance of membranes. Based on our discussion of these influencing factors, there is now a good understanding of the evolution of membrane microstructures and how to control membrane microstructures according to the application purpose. In addition, the thermal stability, oxidation resistance, hydrothermal stability, and chemical resistance of the SiC membranes are described. Due to their robust advantages and high separation performance, SiC membranes are the most promising candidates for high-temperature gas separation. Overall, this review will provide meaningful insight and guidance for developing SiC membranes and achieving excellent gas separation performance.
Keywords: CVD/CVI; gas separation; inorganic membrane; membrane stability; precursor-derived ceramics; silicon carbide membrane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- Voutchkov N. Energy use for membrane seawater desalination—Current status and trends. Desalination. 2018;431:2–14. doi: 10.1016/j.desal.2017.10.033. - DOI
-
- Liang C.Z., Chung T.-S., Lai J.-Y. A review of polymeric composite membranes for gas separation and energy production. Prog. Polym. Sci. 2019;97:101141. doi: 10.1016/j.progpolymsci.2019.06.001. - DOI
Publication types
Grants and funding
- KJ2021A1015/University Natural Sciences Research Project of Anhui Province
- JPMJTM20R7/JST Adaptable and Seamless Technology Transfer Program through Target-driven R&D (A-STEP)
- BK20220002/Natural Science Foundation of Jiangsu Province
- KL21-04/State Key Laboratory of Materials-Oriented Chemical Engineering
- 20RC33/Talent Scientific Research Foundation of Hefei University
LinkOut - more resources
Full Text Sources

