Structural Role of Plasma Membrane Sterols in Osmotic Stress Tolerance of Yeast Saccharomyces cerevisiae
- PMID: 36557185
- PMCID: PMC9781751
- DOI: 10.3390/membranes12121278
Structural Role of Plasma Membrane Sterols in Osmotic Stress Tolerance of Yeast Saccharomyces cerevisiae
Abstract
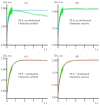
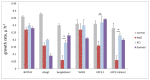
Yeast S. cerevisiae has been shown to suppress a sterol biosynthesis as a response to hyperosmotic stress. In the case of sodium stress, the failure to suppress biosynthesis leads to an increase in cytosolic sodium. The major yeast sterol, ergosterol, is known to regulate functioning of plasma membrane proteins. Therefore, it has been suggested that the suppression of its biosynthesis is needed to adjust the activity of the plasma membrane sodium pumps and channels. However, as the sterol concentration is in the range of thirty to forty percent of total plasma membrane lipids, it is believed that its primary biological role is not regulatory but structural. Here we studied how lowering the sterol content affects the response of a lipid bilayer to an osmotic stress. In accordance with previous observations, we found that a decrease of the sterol fraction increases a water permeability of the liposomal membranes. Yet, we also found that sterol-free giant unilamellar vesicles reduced their volume during transient application of the hyperosmotic stress to a greater extent than the sterol-rich ones. Furthermore, our data suggest that lowering the sterol content in yeast cells allows the shrinkage to prevent the osmotic pressure-induced plasma membrane rupture. We also found that mutant yeast cells with the elevated level of sterol accumulated propidium iodide when exposed to mild hyperosmotic conditions followed by hypoosmotic stress. It is likely that the decrease in a plasma membrane sterol content stimulates a drop in cell volume under hyperosmotic stress, which is beneficial in the case of a subsequent hypo-osmotic one.
Keywords: giant unilamellar vesicle; hyperosmotic stress; hypoosmotic stress; large unilamellar vesicle; light scattering; sterol; yeast.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

