Polyhexanide-Releasing Membranes for Antimicrobial Wound Dressings: A Critical Review
- PMID: 36557188
- PMCID: PMC9781366
- DOI: 10.3390/membranes12121281
Polyhexanide-Releasing Membranes for Antimicrobial Wound Dressings: A Critical Review
Abstract
The prevalence of chronic, non-healing skin wounds in the general population, most notably diabetic foot ulcers, venous leg ulcers and pressure ulcers, is approximately 2% and is expected to increase, driven mostly by the aging population and the steady rise in obesity and diabetes. Non-healing wounds often become infected, increasing the risk of life-threatening complications, which poses a significant socioeconomic burden. Aiming at the improved management of infected wounds, a variety of wound dressings that incorporate antimicrobials (AMDs), namely polyhexanide (poly(hexamethylene biguanide); PHMB), have been introduced in the wound-care market. However, many wound-care professionals agree that none of these wound dressings show comprehensive or optimal antimicrobial activity. This manuscript summarizes and discusses studies on PHMB-releasing membranes (PRMs) for wound dressings, detailing their preparation, physical properties that are relevant to the context of AMDs, drug loading and release, antibacterial activity, biocompatibility, wound-healing capacity, and clinical trials conducted. Some of these PRMs were able to improve wound healing in in vivo models, with no associated cytotoxicity, but significant differences in study design make it difficult to compare overall efficacies. It is hoped that this review, which includes, whenever available, international standards for testing AMDs, will provide a framework for future studies.
Keywords: PHMB; antimicrobial; controlled drug release; cytotoxicity; membrane; poly(hexamethylene biguanide); polyhexamethylene biguanide; polyhexanide; wound dressing; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

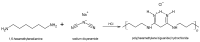
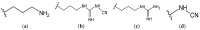




Similar articles
-
Sterilized Polyhexanide-Releasing Chitosan Membranes with Potential for Use in Antimicrobial Wound Dressings.Membranes (Basel). 2023 Nov 8;13(11):877. doi: 10.3390/membranes13110877. Membranes (Basel). 2023. PMID: 37999363 Free PMC article.
-
Polyhexamethylene Biguanide:Polyurethane Blend Nanofibrous Membranes for Wound Infection Control.Polymers (Basel). 2019 May 22;11(5):915. doi: 10.3390/polym11050915. Polymers (Basel). 2019. PMID: 31121845 Free PMC article.
-
Reducing the pathogen burden and promoting healing with polyhexanide in non-healing wounds: a prospective study.J Wound Care. 2015 Dec;24(12):582-6. doi: 10.12968/jowc.2015.24.12.582. J Wound Care. 2015. PMID: 26654738 Clinical Trial.
-
Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration.Health Technol Assess. 2000;4(21):1-237. Health Technol Assess. 2000. PMID: 11074391 Review.
-
Clinical use of 0.1% polyhexanide and propylbetaine on acute and hard-to-heal wounds: a literature review.J Wound Care. 2024 Jun 1;33(Sup6a):cxl-cli. doi: 10.12968/jowc.2019.0066. J Wound Care. 2024. PMID: 38850544 Review.
Cited by
-
Surface-Modified Carboxylated Cellulose Nanofiber Hydrogels for Prolonged Release of Polyhexamethylene Biguanide Hydrochloride (PHMB) for Antimicrobial Applications.Polymers (Basel). 2023 Aug 28;15(17):3572. doi: 10.3390/polym15173572. Polymers (Basel). 2023. PMID: 37688198 Free PMC article.
-
Development and Evaluation of a Novel Antibacterial Wound Dressing: A Powder Preparation Based on Cross-Linked Pullulan with Polyhexamethylene Biguanide for Hydrogel-Transition in Advanced Wound Management and Infection Control.Polymers (Basel). 2024 May 10;16(10):1352. doi: 10.3390/polym16101352. Polymers (Basel). 2024. PMID: 38794544 Free PMC article.
-
Tissue Regeneration of Radiation-Induced Skin Damages Using Protein/Polysaccharide-Based Bioengineered Scaffolds and Adipose-Derived Stem Cells: A Review.Int J Mol Sci. 2025 Jul 4;26(13):6469. doi: 10.3390/ijms26136469. Int J Mol Sci. 2025. PMID: 40650244 Free PMC article. Review.
-
Current status and progress in research on dressing management for diabetic foot ulcer.Front Endocrinol (Lausanne). 2023 Aug 17;14:1221705. doi: 10.3389/fendo.2023.1221705. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37664860 Free PMC article. Review.
-
Comparison of In Vitro Bacterial Susceptibility to Common and Novel Equine Wound Care Dressings.Animals (Basel). 2024 Mar 1;14(5):776. doi: 10.3390/ani14050776. Animals (Basel). 2024. PMID: 38473161 Free PMC article.
References
-
- Gray D., Barrett S., Battacharyya M., Butcher M., Enoch S., Fumerola S., Stephen-Haynes J., Edwards-Jones V., Leaper D., Strohal R., et al. PHMB and its potential contribution to wound management. Wounds. 2010;6:40–46.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

