A Validated HPLC-Diode Array Detection Method for Therapeutic Drug Monitoring of Thiopurines in Pediatric Patients: From Bench to Bedside
- PMID: 36557210
- PMCID: PMC9785603
- DOI: 10.3390/metabo12121173
A Validated HPLC-Diode Array Detection Method for Therapeutic Drug Monitoring of Thiopurines in Pediatric Patients: From Bench to Bedside
Abstract
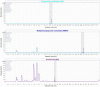
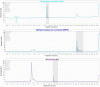
Thiopurine drugs are part of the therapeutic armamentarium for pediatric patients suffering from inflammatory bowel disease (IBD) and acute lymphoblastic leukemia (ALL). The therapeutic drug monitoring of these drugs, consisting of measurements of the thiopurine metabolites thioguanine nucleotides (TGN) and methylmercaptopurine nucleotides (MMPN) are used to optimize the effectiveness of treatment and prevent adverse effects. In this context, we developed and validated a high-performance liquid chromatography-diode array detection (HPLC-DAD) method for the simultaneous quantification of thiopurine metabolites according to the most recent International Council for Harmonisation (ICH) guidelines. The calibration curves were built in the clinically relevant range of concentrations for TGN of 300-12,000 nM and for MMPN of 3000-60,000 nM. The limit of detection and the lower limit of quantification were 100 and 300 nM for TGN and 900 and 3000 nM for MMPN, respectively. The percentage of inter-day accuracy and precision (CV%) varied between 85 and 104% and 1.6 and 13.8%. Stability was demonstrated for both of the metabolites for at least 50 days at -20 °C. The proposed HPLC-DAD method showed an appropriate selectivity, specificity, linearity, accuracy, precision and good applicability to samples from patients with IBD and ALL undergoing thiopurine treatment.
Keywords: pediatric patients; therapeutic drug monitoring; thiopurines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Van Rheenen P.F., Aloi M., Assa A., Bronsky J., Escher J.C., Fagerberg U.L., Gasparetto M., Gerasimidis K., Griffiths A., Henderson P., et al. The Medical Management of Paediatric Crohn’s Disease: An ECCO-ESPGHAN Guideline Update. J. Crohn’s Colitis. 2020;15:171–194. doi: 10.1093/ecco-jcc/jjaa161. - DOI - PubMed
-
- Turner D., Ruemmele F.M., Orlanski-Meyer E., Griffiths A.M., de Carpi J.M., Bronsky J., Veres G., Aloi M., Strisciuglio C., Braegger C.P., et al. Management of Paediatric Ulcerative Colitis, Part 1: Ambulatory Care—An Evidence-based Guideline from European Crohn’s and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J. Craniofacial Surg. 2018;67:257–291. doi: 10.1097/MPG.0000000000002035. - DOI - PubMed
-
- Franca R., Stocco G., Favretto D., Giurici N., Del Rizzo I., Locatelli F., Vinti L., Biondi A., Colombini A., Fagioli F., et al. PACSIN2 rs2413739 influence on thiopurine pharmacokinetics: Validation studies in pediatric patients. Pharm. J. 2019;20:415–425. doi: 10.1038/s41397-019-0130-0. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

