Urinary Metabolic Profiling in Volunteers Undergoing Malaria Challenge in Gabon
- PMID: 36557262
- PMCID: PMC9783708
- DOI: 10.3390/metabo12121224
Urinary Metabolic Profiling in Volunteers Undergoing Malaria Challenge in Gabon
Abstract
The interaction of malaria parasites with their human host is extensively studied, yet only few studies reported how P. falciparum infection affects urinary metabolite profiles and how this is associated with immunity. We present a longitudinal study of the urinary metabolic profiles of twenty healthy Africans with lifelong exposure to malaria and five malaria-naïve Europeans, who were all challenged with direct venous inoculation of live P. falciparum sporozoïtes (PfSPZ) and followed up until they developed symptoms or became thick blood smear positive (TBS). Urine samples were collected before and at 2, 5, 9 and 11 days post challenge and were analysed. Upon infection, all Europeans became TBS positive, while Africans showed either a delay in time to parasitaemia or controlled infection. Our metabolic data showed that Europeans and Africans had distinct alterations in metabolite patterns, with changes mostly seen on days 5 and 9 post PfSPZ infection, and more prominently in Europeans. Within the African group, the levels of formate, urea, trimethylamine, threonine, choline, myo-inositol and acetate were significantly higher in TBS positive whereas the levels of pyruvate, 3-methylhistidine and dimethylglycine were significantly lower in individuals who remained TBS negative. Notably, before inoculation with PfSPZ, a group of metabolites including phenylacetylglutamine can potentially be used to predict parasitaemia control among Africans. Taken together, this study highlights the difference in urinary metabolic changes in response to malaria infection as a consequence of lifelong exposure to malaria and that change detectable before challenge might predict the control of parasitaemia in malaria-endemic areas.
Keywords: Gabon; Plasmodium falciparum malaria; hydrophilic interaction chromatography-mass spectrometry; metabolomics; nuclear magnetic resonance; urine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
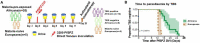





References
-
- Arya A., Foko L.P.K., Chaudhry S., Sharma A., Singh V. Artemisinin-based combination therapy (ACT) and drug resistance molecular markers: A systematic review of clinical studies from two malaria endemic regions—India and sub-Saharan Africa. Int. J. Parasitol. Drugs Drug Resist. 2021;15:43–56. doi: 10.1016/j.ijpddr.2020.11.006. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

