Antibacterial and Antibiofilm Activity of Carvacrol against Oral Pathogenic Bacteria
- PMID: 36557293
- PMCID: PMC9785330
- DOI: 10.3390/metabo12121255
Antibacterial and Antibiofilm Activity of Carvacrol against Oral Pathogenic Bacteria
Abstract
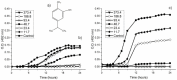
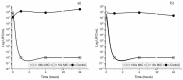
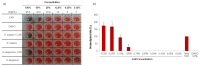
Faced with the current situation of high rates of microbial resistance, together with the scarcity of new antibiotics, it is necessary to search for and identify new antimicrobials, preferably natural, to alleviate this situation. The aim of this work was to evaluate the antibacterial activity of carvacrol (CAR), a phenolic compound of essential oils, against pathogenic microorganisms causing oral infections, such as Streptococcus mutans and S. sanguinis, never evaluated before. The minimum inhibitory and the minimum bactericidal concentration were 93.4 μg/mL and 373.6 μg/mL, respectively, for the two strains. The growth kinetics under different concentrations of CAR, as well as the bactericidal power were determined. The subinhibitory concentrations delayed and decreased bacterial growth. Its efficacy on mature biofilms was also tested. Finally, the possible hemolytic effect of CAR, not observable at the bactericidal concentrations under study, was evaluated. Findings obtained point to CAR as an excellent alternative agent to safely prevent periodontal diseases. In addition, it is important to highlight the use of an experimental methodology that includes dual-species biofilm and subinhibitory concentration models to determine optimal CAR treatment concentrations. Thus, CAR could be used preventively in mouthwashes or biomaterials, or in treatments to avoid existing antibiotic resistance.
Keywords: Streptococcus; antibacterial activity; biofilm; carvacrol; oral diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

