In Vitro, In Silico and Network Pharmacology Mechanistic Approach to Investigate the α-Glucosidase Inhibitors Identified by Q-ToF-LCMS from Phaleria macrocarpa Fruit Subcritical CO2 Extract
- PMID: 36557305
- PMCID: PMC9783102
- DOI: 10.3390/metabo12121267
In Vitro, In Silico and Network Pharmacology Mechanistic Approach to Investigate the α-Glucosidase Inhibitors Identified by Q-ToF-LCMS from Phaleria macrocarpa Fruit Subcritical CO2 Extract
Abstract
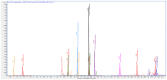
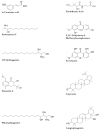
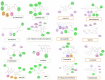
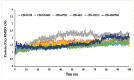
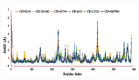
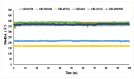
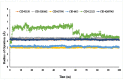
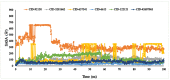
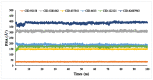
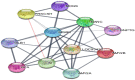
The fruit of Phaleria macrocarpa have been traditionally used as an antidiabetic remedy in Malaysia and neighbouring countries. Despite its potential for diabetes treatment, no scientific study has ever been conducted to predict the inhibitor interaction of the protein α-glucosidase identified in an extract prepared with a non-conventional extraction technique. Hence, the major aim of this research was to evaluate the in vitro antioxidant, the α-glucosidase inhibitors, and the molecular dynamic simulations of the α-glucosidase inhibitors identified by Quadrupole Time-of-Flight Liquid Chromatography Mass Spectrometry (Q-ToF-LCMS) analysis. Initially, dry fruit were processed using non-conventional and conventional extraction methods to obtain subcritical carbon dioxide extracts (SCE-1 and SCE-2) and heating under reflux extract (HRE), respectively. Subsequently, all extracts were evaluated for their in vitro antioxidative and α-glucosidase inhibitory potentials. Subsequently, the most bioactive extract (SCE-2) was subjected to Q-ToF-LCMS analysis to confirm the presence of α-glucosidase inhibitors, which were then analysed through molecular dynamic simulations and network pharmacology approaches to confirm their possible mechanism of action. The highest inhibitory effects of the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical and α-glucosidase on SCE-2 was found as 75.36 ± 0.82% and 81.79 ± 0.82%, respectively, compared to the SCE-1 and HRE samples. The Q-ToF-LCMS analysis tentatively identified 14 potent α-glucosidase inhibitors. Finally, five identified compounds, viz., lupenone, swertianolin, m-coumaric acid, pantothenic acid, and 8-C-glucopyranosyleriodictylol displayed significant stability, compactness, stronger protein-ligand interaction up to 100 ns further confirming their potential as α-glucosidase inhibitors. Consequently, it was concluded that the SCE-2 possesses a strong α-glucosidase inhibitory effect due to the presence of these compounds. The findings of this study might prove useful to develop these compounds as alternative safe α-glucosidase inhibitors to manage diabetes more effectively.
Keywords: Phaleria macrocarpa fruit; bioactive compounds; molecular dynamic simulations; network pharmacology; subcritical CO2 extract; α-glucosidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Rapid investigation of α-glucosidase inhibitory activity of Phaleria macrocarpa extracts using FTIR-ATR based fingerprinting.J Food Drug Anal. 2017 Apr;25(2):306-315. doi: 10.1016/j.jfda.2016.09.007. Epub 2016 Nov 5. J Food Drug Anal. 2017. PMID: 28911672 Free PMC article.
-
In vitro and in vivo effects of standardized extract and fractions of Phaleria macrocarpa fruits pericarp on lead carbohydrate digesting enzymes.BMC Complement Altern Med. 2013 Feb 20;13:39. doi: 10.1186/1472-6882-13-39. BMC Complement Altern Med. 2013. PMID: 23425283 Free PMC article.
-
Identification of putative α-glucosidase inhibitors and antioxidants in Zingiber officinale rhizome using LCMS-based metabolomics and in silico molecular docking.Nat Prod Res. 2024 Jun 25:1-6. doi: 10.1080/14786419.2024.2369224. Online ahead of print. Nat Prod Res. 2024. PMID: 38919043
-
An activity-integrated strategy involving ultra-high-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry and fraction collector for rapid screening and characterization of the α-glucosidase inhibitors in Coptis chinensis Franch. (Huanglian).J Pharm Biomed Anal. 2014 Nov;100:79-87. doi: 10.1016/j.jpba.2014.07.025. Epub 2014 Aug 1. J Pharm Biomed Anal. 2014. PMID: 25137652
-
Phaleria macrocarpa (Scheff.) Boerl.: An updated review of pharmacological effects, toxicity studies, and separation techniques.Saudi Pharm J. 2023 Jun;31(6):874-888. doi: 10.1016/j.jsps.2023.04.006. Epub 2023 Apr 12. Saudi Pharm J. 2023. PMID: 37234341 Free PMC article. Review.
Cited by
-
In silico-based investigation of the molecular mechanism of Artocarpus communis seed hexane fraction against metabolic syndrome.J Mol Model. 2025 Jan 23;31(2):60. doi: 10.1007/s00894-024-06274-8. J Mol Model. 2025. PMID: 39847117
References
-
- Kumar Thakur A., Kumar Y., Goyal R.K. Pharmacotherapeutics of miglitol: An α-glucosidase inhibitor. J. Anal. Pharm. Res. 2018;7:617–619. doi: 10.15406/japlr.2018.07.00292. - DOI
-
- Brownlee I.A., Gill S., Wilcox M.D., Pearson J.P., Chater P.I. Starch digestion in the upper gastrointestinal tract of humans. Starch-Starke. 2018;70:1700111. doi: 10.1002/star.201700111. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

