Optimising Fluvoxamine Maternal/Fetal Exposure during Gestation: A Pharmacokinetic Virtual Clinical Trials Study
- PMID: 36557319
- PMCID: PMC9782298
- DOI: 10.3390/metabo12121281
Optimising Fluvoxamine Maternal/Fetal Exposure during Gestation: A Pharmacokinetic Virtual Clinical Trials Study
Abstract
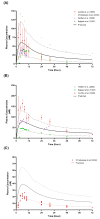
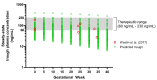
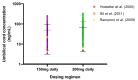
Fluvoxamine plasma concentrations have been shown to decrease throughout pregnancy. CYP2D6 polymorphisms significantly influence these changes. However, knowledge of an optimum dose adjustment according to the CYP2D6 phenotype is still limited. This study implemented a physiologically based pharmacokinetic modelling approach to assess the gestational changes in fluvoxamine maternal and umbilical cord concentrations. The optimal dosing strategies during pregnancy were simulated, and the impact of CYP2D6 phenotypes on fluvoxamine maternal and fetal concentrations was considered. A significant decrease in fluvoxamine maternal plasma concentrations was noted during gestation. As for the fetal concentration, a substantial increase was noted for the poor metabolisers (PM), with a constant level in the ultrarapid (UM) and extensive (EM) metabolisers commencing from gestation week 20 to term. The optimum dosing regimen suggested for UM and EM reached a maximum dose of 300 mg daily at gestational weeks (GW) 15 and 35, respectively. In contrast, a stable dose of 100 mg daily throughout gestation for the PM is sufficient to maintain the fluvoxamine plasma concentration within the therapeutic window (60-230 ng/mL). Dose adjustment during pregnancy is required for fluvoxamine, particularly for UM and EM, to maintain efficacy throughout the gestational period.
Keywords: depression; fetal; fluvoxamine; pharmacokinetics; pregnancy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Precision dosing-based optimisation of paroxetine during pregnancy for poor and ultrarapid CYP2D6 metabolisers: a virtual clinical trial pharmacokinetics study.J Pharm Pharmacol. 2020 Aug;72(8):1049-1060. doi: 10.1111/jphp.13281. Epub 2020 Apr 28. J Pharm Pharmacol. 2020. PMID: 32346890
-
Precision dosing of venlafaxine during pregnancy: a pharmacokinetics modelling approach.J Pharm Pharmacol. 2024 Jan 27;76(2):122-137. doi: 10.1093/jpp/rgad106. J Pharm Pharmacol. 2024. PMID: 38142123
-
Precision dosing of methadone during pregnancy: A pharmacokinetics virtual clinical trials study.J Subst Abuse Treat. 2021 Nov;130:108521. doi: 10.1016/j.jsat.2021.108521. Epub 2021 Jun 3. J Subst Abuse Treat. 2021. PMID: 34118695 Clinical Trial.
-
Pharmacokinetic-pharmacodynamic relationship of the selective serotonin reuptake inhibitors.Clin Pharmacokinet. 1996 Dec;31(6):444-69. doi: 10.2165/00003088-199631060-00004. Clin Pharmacokinet. 1996. PMID: 8968657 Review.
-
Clinical pharmacokinetics of fluvoxamine: applications to dosage regimen design.J Clin Psychiatry. 1997;58 Suppl 5:7-14. J Clin Psychiatry. 1997. PMID: 9184622 Review.
Cited by
-
The Impact of Paediatric Obesity on Drug Pharmacokinetics: A Virtual Clinical Trials Case Study with Amlodipine.Pharmaceutics. 2024 Apr 2;16(4):489. doi: 10.3390/pharmaceutics16040489. Pharmaceutics. 2024. PMID: 38675150 Free PMC article.
-
An Overview of the Systematic Reviews About the Efficacy of Fluvoxamine on Depression.Pharmaceuticals (Basel). 2025 May 12;18(5):711. doi: 10.3390/ph18050711. Pharmaceuticals (Basel). 2025. PMID: 40430530 Free PMC article. Review.
References
-
- Hiemke C., Bergemann N., Clement H.W., Conca A., Deckert J., Domschke K., Eckermann G., Egberts K., Gerlach M., Greiner C., et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: Update 2017. Pharmacopsychiatry. 2018;51:e1. doi: 10.1055/s-0037-1600991. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

