An Efficient and Economical N-Glycome Sample Preparation Using Acetone Precipitation
- PMID: 36557323
- PMCID: PMC9786591
- DOI: 10.3390/metabo12121285
An Efficient and Economical N-Glycome Sample Preparation Using Acetone Precipitation
Abstract
Due to the critical role of the glycome in organisms and its close connections with various diseases, much time and effort have been dedicated to glycomics-related studies in the past decade. To achieve accurate and reliable identification and quantification of glycans extracted from biological samples, several analysis methods have been well-developed. One commonly used methodology for the sample preparation of N-glycomics usually involves enzymatic cleavage by PNGase F, followed by sample purification using C18 cartridges to remove proteins. PNGase F and C18 cartridges are very efficient both for cleaving N-glycans and for protein removal. However, this method is most suitable for a limited quantity of samples. In this study, we developed a sample preparation method focusing on N-glycome extraction and purification from large-scale biological samples using acetone precipitation. The N-glycan yield was first tested on standard glycoprotein samples, bovine fetuin and complex biological samples, and human serum. Compared to C18 cartridges, most of the sialylated N-glycans from human serum were detected with higher abundance after acetone precipitation. However, C18 showed a slightly higher efficiency for protein removal. Using the unfiltered human serum as the baseline, around 97.7% of the proteins were removed by acetone precipitation, while more than 99.9% of the proteins were removed by C18 cartridges. Lastly, the acetone precipitation was applied to N-glycome extraction from egg yolks to demonstrate large-scale glycomics sample preparation.
Keywords: LC-MS/MS; acetone precipitation; glycan; oxidative release; permethylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
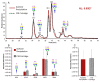
 , N-acetylglucosamine
(GlcNAc);
, N-acetylglucosamine
(GlcNAc);  , Galactose (Gal);
, Galactose (Gal);  , Fucose (Fuc);
, Fucose (Fuc);  , Mannose (Man);
, Mannose (Man);  , N-acetylneuraminic
acid (NeuAc/Sialic Acid).
, N-acetylneuraminic
acid (NeuAc/Sialic Acid).

Similar articles
-
Large-scale quantitative isolation of pure protein N-linked glycans.Carbohydr Res. 2019 Jun 1;479:13-22. doi: 10.1016/j.carres.2019.04.011. Epub 2019 May 3. Carbohydr Res. 2019. PMID: 31100702
-
Providing Bionic Glycome as internal standards by glycan reducing and isotope labeling for reliable and simple quantitation of N-glycome based on MALDI- MS.Anal Chim Acta. 2019 Nov 12;1081:112-119. doi: 10.1016/j.aca.2019.07.003. Epub 2019 Jul 3. Anal Chim Acta. 2019. PMID: 31446948
-
Polysialylated N-glycans identified in human serum through combined developments in sample preparation, separations, and electrospray ionization-mass spectrometry.Anal Chem. 2014 Sep 2;86(17):8700-10. doi: 10.1021/ac501839b. Epub 2014 Aug 21. Anal Chem. 2014. PMID: 25118826 Free PMC article.
-
Quantitative O-glycomics based on improvement of the one-pot method for nonreductive O-glycan release and simultaneous stable isotope labeling with 1-(d0/d5)phenyl-3-methyl-5-pyrazolone followed by mass spectrometric analysis.J Proteomics. 2017 Jan 6;150:18-30. doi: 10.1016/j.jprot.2016.08.012. Epub 2016 Aug 29. J Proteomics. 2017. PMID: 27585995
-
Preparation of glycopeptides by acetone precipitation.2021 Sep 15 [updated 2022 Mar 14]. In: Nishihara S, Angata K, Aoki-Kinoshita KF, Hirabayashi J, editors. Glycoscience Protocols (GlycoPODv2) [Internet]. Saitama (JP): Japan Consortium for Glycobiology and Glycotechnology; 2021–. 2021 Sep 15 [updated 2022 Mar 14]. In: Nishihara S, Angata K, Aoki-Kinoshita KF, Hirabayashi J, editors. Glycoscience Protocols (GlycoPODv2) [Internet]. Saitama (JP): Japan Consortium for Glycobiology and Glycotechnology; 2021–. PMID: 37590573 Free Books & Documents. Review. No abstract available.
Cited by
-
Analysis of carbohydrates and glycoconjugates by matrix-assisted laser desorption/ionization mass spectrometry: An update for 2021-2022.Mass Spectrom Rev. 2025 May-Jun;44(3):213-453. doi: 10.1002/mas.21873. Epub 2024 Jun 24. Mass Spectrom Rev. 2025. PMID: 38925550 Free PMC article. Review.
-
Effects of Intracellular Polysaccharides and Proteins of Auxenochlorella pyrenoidosa on Water Quality, Floc Formation, and Microbial Composition in a Biofloc System.Microorganisms. 2025 Jul 21;13(7):1704. doi: 10.3390/microorganisms13071704. Microorganisms. 2025. PMID: 40732213 Free PMC article.
-
Suppressing the background of LC-ESI-MS analysis of permethylated glycans using the active background ion reduction device.Electrophoresis. 2024 Sep;45(17-18):1469-1478. doi: 10.1002/elps.202300301. Epub 2024 Apr 4. Electrophoresis. 2024. PMID: 38573014 Free PMC article.
-
LC-MS/MS of isomeric N-and O-glycopeptides on mesoporous graphitized carbon column.Anal Chim Acta. 2024 Aug 15;1317:342907. doi: 10.1016/j.aca.2024.342907. Epub 2024 Jun 25. Anal Chim Acta. 2024. PMID: 39030008 Free PMC article.
-
Metabolomic Changes in Rat Serum after Chronic Exposure to Glyphosate-Based Herbicide.Metabolites. 2024 Jan 13;14(1):50. doi: 10.3390/metabo14010050. Metabolites. 2024. PMID: 38248853 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

