PafS Containing GGDEF-Domain Regulates Life Activities of Pseudomonas glycinae MS82
- PMID: 36557595
- PMCID: PMC9781394
- DOI: 10.3390/microorganisms10122342
PafS Containing GGDEF-Domain Regulates Life Activities of Pseudomonas glycinae MS82
Abstract
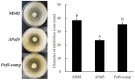
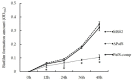
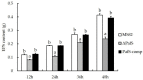
Cyclic dimeric guanosine monophosphate (c-di-GMP) is synthesized by diguanylate cyclase (DGC) with the GGDEF domain. As a ubiquitous bacterial second messenger, it regulates diverse life-activity phenotypes in some bacteria. Although 38 genes encoding GGDEF-domain-containing proteins have been identified in the genome of the Pseudomonas glycinae strain MS82, whether c-di-GMP functions as a facilitator or repressor of life-activity phenotypes is poorly understood. In this study, one of the 38 genes containing a GGDEF domain in MS82, PafS was investigated to explore its regulatory function in bacterial life activities. The PafS-deletion mutant ΔPafS and reversion mutant PafS-comp were constructed by the method of biparental conjugation and homologous recombination. The life activities of the mutants, such as antifungal activity, biofilm formation ability, polysaccharide content, and motor behavior, were explored. The results showed that all life-activity phenotypes were significantly reduced after knocking out PafS, whereas all were significantly restored to a similar level to that of MS82 after the complementation of PafS. These results suggested that PafS plays an important role in the regulation of a range of cellular activities by c-di-GMP in P. glycinae MS82.
Keywords: GGDEF domain; PafS; Pseudomonas glycinae MS82; life activities.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Diguanylate Cyclase GdpX6 with c-di-GMP Binding Activity Involved in the Regulation of Virulence Expression in Xanthomonas oryzae pv. oryzae.Microorganisms. 2021 Feb 26;9(3):495. doi: 10.3390/microorganisms9030495. Microorganisms. 2021. PMID: 33652966 Free PMC article.
-
The GGDEF-domain protein GdpX1 attenuates motility, exopolysaccharide production and virulence in Xanthomonas oryzae pv. oryzae.J Appl Microbiol. 2016 Jun;120(6):1646-57. doi: 10.1111/jam.13115. Epub 2016 Apr 4. J Appl Microbiol. 2016. PMID: 26929398
-
More than Enzymes That Make or Break Cyclic Di-GMP-Local Signaling in the Interactome of GGDEF/EAL Domain Proteins of Escherichia coli.mBio. 2017 Oct 10;8(5):e01639-17. doi: 10.1128/mBio.01639-17. mBio. 2017. PMID: 29018125 Free PMC article.
-
C-di-GMP Synthesis: Structural Aspects of Evolution, Catalysis and Regulation.J Mol Biol. 2016 Sep 25;428(19):3683-701. doi: 10.1016/j.jmb.2016.07.023. Epub 2016 Aug 4. J Mol Biol. 2016. PMID: 27498163 Review.
-
Screening for Diguanylate Cyclase (DGC) Inhibitors Mitigating Bacterial Biofilm Formation.Front Chem. 2020 Apr 21;8:264. doi: 10.3389/fchem.2020.00264. eCollection 2020. Front Chem. 2020. PMID: 32373581 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

