CD36-A Host Receptor Necessary for Malaria Parasites to Establish and Maintain Infection
- PMID: 36557610
- PMCID: PMC9785914
- DOI: 10.3390/microorganisms10122356
CD36-A Host Receptor Necessary for Malaria Parasites to Establish and Maintain Infection
Abstract
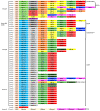
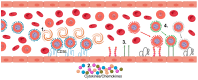
Plasmodium falciparum-infected erythrocytes (PfIEs) present P. falciparum erythrocyte membrane protein 1 proteins (PfEMP1s) on the cell surface, via which they cytoadhere to various endothelial cell receptors (ECRs) on the walls of human blood vessels. This prevents the parasite from passing through the spleen, which would lead to its elimination. Each P. falciparum isolate has about 60 different PfEMP1s acting as ligands, and at least 24 ECRs have been identified as interaction partners. Interestingly, in every parasite genome sequenced to date, at least 75% of the encoded PfEMP1s have a binding domain for the scavenger receptor CD36 widely distributed on host endothelial cells and many other cell types. Here, we discuss why the interaction between PfIEs and CD36 is optimal to maintain a finely regulated equilibrium that allows the parasite to multiply and spread while causing minimal harm to the host in most infections.
Keywords: CD36; Plasmodium falciparum; cytoadhesion; endothelial cell receptor; malaria; sequestration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- WHO . World Malaria Report 2021. WHO; Geneva, Switzerland: 2021.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

