Anti-Inflammatory and Antibacterial Effects and Mode of Action of Greek Arbutus, Chestnut, and Fir Honey in Mouse Models of Inflammation and Sepsis
- PMID: 36557628
- PMCID: PMC9784341
- DOI: 10.3390/microorganisms10122374
Anti-Inflammatory and Antibacterial Effects and Mode of Action of Greek Arbutus, Chestnut, and Fir Honey in Mouse Models of Inflammation and Sepsis
Abstract
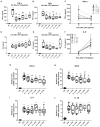
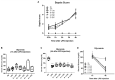
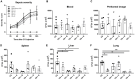
Background: Honey has been shown to possess anti-inflammatory and bactericidal properties that may be useful for the prevention and treatment of infections as well as of acute and chronic inflammatory diseases. The antimicrobial potency of honey could be attributed to its physicochemical characteristics combined with the presence of certain compounds, such as hydrogen peroxide and polyphenols. Honey's bacteriostatic or bactericidal capacity varies depending on its composition and the bacterial type of each infection. Nevertheless, not all honey samples possess anti-inflammatory or antibacterial properties and their mechanism of action has not been clearly elucidated. Objectives: We therefore investigated the anti-inflammatory properties of three different honey samples that derived from different geographical areas of Greece and different botanical origins, namely, arbutus, chestnut, and fir; they were compared to manuka honey, previously known for its anti-inflammatory and antibacterial activity. Materials and Methods: To test the anti-inflammatory activity of the different samples, we utilized the in vivo model of LPS-driven inflammation, which induces septic shock without the presence of pathogens. To evaluate the antibacterial action of the same honey preparations, we utilized the cecal-slurry-induced peritonitis model in mice. Since acute inflammation and sepsis reduce the biotransformation capacity of the liver, the expression of key enzymes in the process was also measured. Results: The administration of all Greek honey samples to LPS-stimulated mice revealed a potent anti-inflammatory activity by suppressing the TNFα serum levels and the expression of TNFα and iNOS in the liver at levels comparable to those of the manuka honey, but they had no effect on IL-6 or IL-1β. It was shown that the LPS-induced suppression of CYP1A1 in the liver was reversed by Epirus and Crete fir honey, while, correspondingly, the suppression of CYP2B10 in the liver was reversed by Evros chestnut and Epirus fir honey. The effect of the same honey samples in polymicrobial peritonitis in mice was also evaluated. Even though no effect was observed on the disease severity or peritoneal bacterial load, the bacterial load in the liver was reduced in mice treated with Evros chestnut, Epiros fir, and Crete fir, while the bacterial load in the lungs was reduced in Epirus arbutus, Crete fir, and manuka honey-treated mice. Conclusion: Our findings suggest that these specific Greek honey samples possess distinct anti-inflammatory and antibacterial properties, as evidenced by the reduced production of pro-inflammatory mediators and the impaired translocation of bacteria to tissues in septic mice. Their mode of action was comparable or more potent to those of manuka honey.
Keywords: LPS; Manuka; TNF-a; antimicrobial; bactericidal; cecal slurry-induced peritonitis; honey; iNOS; inflammation; mice; natural compounds; sepsis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., Colombara D.V., Ikuta K.S., Kissoon N., Finfer S., et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. - DOI - PMC - PubMed
-
- El-Seedi H.R., Eid N., Abd El-Wahed A.A., Rateb M.E., Afifi H.S., Algethami A.F., Zhao C., Al Naggar Y., Alsharif S.M., Tahir H.E., et al. Honey Bee Products: Preclinical and Clinical Studies of Their Anti-inflammatory and Immunomodulatory Properties. Front. Nutr. 2022;8:761267. doi: 10.3389/fnut.2021.761267. - DOI - PMC - PubMed
-
- Combarros-Fuertes P., Estevinho L.M., Dias L.G., Castro J.M., Tomás-Barberán F.A., Tornadijo M.E., Fresno-Baro J.M. Bioactive Components and Antioxidant and Antibacterial Activities of Different Varieties of Honey: A Screening Prior to Clinical Application. J. Agric. Food Chem. 2019;67:688–698. doi: 10.1021/acs.jafc.8b05436. - DOI - PubMed
-
- El-Kazafy A.T., Saad A.-K., Reda T. Comparison of Pollen Spectra and Amount of Mineral Content in Honey Produced by Apis florea F. and Apis mellifera L. J. Kans. Entomol. Soc. 2018;91:51–57. doi: 10.2317/0022-8567-91.1.51. - DOI
LinkOut - more resources
Full Text Sources

