Comparison between Symptomatic and Asymptomatic Mice after Clostridioides difficile Infection Reveals Novel Inflammatory Pathways and Contributing Microbiota
- PMID: 36557633
- PMCID: PMC9782979
- DOI: 10.3390/microorganisms10122380
Comparison between Symptomatic and Asymptomatic Mice after Clostridioides difficile Infection Reveals Novel Inflammatory Pathways and Contributing Microbiota
Abstract
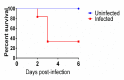
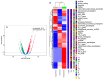
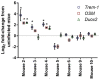
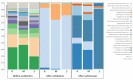
Clostridioides difficile causes the highest number of nosocomial infections. Currently, treatment options for C. difficile infection (CDI) are very limited, resulting in poor treatment outcomes and high recurrence rates. Although the disease caused by CDI is inflammatory in nature, the role of inflammation in the development of CDI symptoms is contradictory and not completely understood. Hence, the use of anti-inflammatory medication is debatable in CDI. In the current study, we evaluated the genetic and microbiome profiles of mice after infection with C. difficile. These mice were categorized based on the severity of CDI and the results were viewed accordingly. Our results indicate that certain genes are upregulated in severe CDI more than in the moderate case. These include oncostatin-M (OSM), matrix metalloprotease 8 (MMP8), triggering receptor expressed on myeloid cells 1 (Trem-1), and dual oxidase 2 (Duox2). We also investigated the microbiome composition of CDI mice before and after infecting with C. difficile. The results show that C. difficile abundance is not indicative of diseases severity. Certain bacterial species (e.g., Citrobacter) were enriched while others (e.g., Turicibacter) were absent in severe CDI. This study identifies novel inflammatory pathways and bacterial species with a potential role in determining the severity of CDI.
Keywords: Citrobacter amalonaticus; Clostridioides difficile; Duox2; MMP; OSM; Trem-1; Turicibacter; inflammation; microbiome; mouse model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- McDonald L.C., Gerding D.N., Johnson S., Bakken J.S., Carroll K.C., Coffin S.E., Dubberke E.R., Garey K.W., Gould C.V., Kelly C., et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2018;66:e1–e48. doi: 10.1093/cid/cix1085. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

