Akkermansia muciniphila and Faecalibacterium prausnitzii in Immune-Related Diseases
- PMID: 36557635
- PMCID: PMC9782003
- DOI: 10.3390/microorganisms10122382
Akkermansia muciniphila and Faecalibacterium prausnitzii in Immune-Related Diseases
Abstract
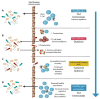
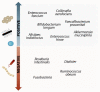
Probiotics and synbiotics are used to treat chronic illnesses due to their roles in immune system modulation and anti-inflammatory response. They have been shown to reduce inflammation in a number of immune-related disorders, including systemic lupus erythematosus (SLE), human immunodeficiency virus (HIV), and chronic inflammatory skin conditions such as psoriasis and atopic dermatitis (AD). Akkermansia muciniphila (A. muciniphila) and Faecalibacterium prausnitzii (F. prausnitzii) are two different types of bacteria that play a significant part in this function. It has been established that Akkermansia and Faecalibacterium are abundant in normal populations and have protective benefits on digestive health while also enhancing the immune system, metabolism, and gut barrier of the host. They have the potential to be a therapeutic target in diseases connected to the microbiota, such as immunological disorders and cancer immunotherapy. There has not been a review of the anti-inflammatory effects of Akkermansia and Faecalibacterium, particularly in immunological diseases. In this review, we highlight the most recent scientific findings regarding A. muciniphila and F. prausnitzii as two significant gut microbiota for microbiome alterations and seek to provide cutting-edge insight in terms of microbiome-targeted therapies as promising preventive and therapeutic tools in immune-related diseases and cancer immunotherapy.
Keywords: Akkermansia muciniphila; Faecalibacterium prausnitzii; atopic dermatitis; human immunodeficiency virus; immune-related diseases; immunotherapy; probiotics; psoriasis; systemic lupus erythematosus.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures
References
-
- Roy P., Kumar V. Functional Food: Probiotic as Health Booster. J. Food Nutr. Popul. Health. 2018;2:12. doi: 10.21767/2577-0586.100042. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

