Three Methods Assessing the Association of the Endophytic Entomopathogenic Fungus Metarhizium robertsii with Non-Grafted Grapevine Vitis vinifera
- PMID: 36557691
- PMCID: PMC9787814
- DOI: 10.3390/microorganisms10122437
Three Methods Assessing the Association of the Endophytic Entomopathogenic Fungus Metarhizium robertsii with Non-Grafted Grapevine Vitis vinifera
Abstract
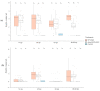
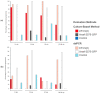
Characterizing the association of endophytic insect pathogenic fungi (EIPF) with plants is an important step in order to understand their ecology before using them in biological control programs. Since several methods are available, it is challenging to identify the most appropriate for such investigations. Here, we used two strains of Metarhizium robertsii: EF3.5(2) native to the French vineyard environment and ARSEF-2575-GFP a laboratory strain expressing a green fluorescent protein, to compare their potential of association with non-grafted grapevine Vitis vinifera. Three methods were used to evaluate the kinetics of rhizosphere and grapevine endospheric colonization: (i) Droplet Digital (ddPCR), a sensitive molecular method of M. robertsii DNA quantification in different plant parts, (ii) culture-based method to detect the live fungal propagules from plant tissues that grew on the medium, (iii) confocal imaging to observe roots segments. Both strains showed evidence of establishment in the rhizosphere of grapevines according to the culture-based and ddPCR methods, with a significantly higher establishment of strain EF3.5(2) (40% positive plants and quantified median of exp(4.61) c/μL) compared to strain ARSEF-2575-GFP (13% positive plants and quantified median of exp(2.25) c/μL) at 96-98 days post-inoculation. A low incidence of association of both strains in the grapevine root endosphere was found with no significant differences between strains and evaluation methods (15% positive plants inoculated with strain EF3.5(2) and 5% with strain ARSEF-2575-GFP according to culture-based method). ddPCR should be used more extensively to investigate the association between plants and EIPF but always accompanied with at least one method such as culture-based method or confocal microscopy.
Keywords: ddPCR; endophytes; fungal entomopathogens; rhizosphere.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Jaber L.R., Ownley B.H. Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biol. Control. 2018;116:36–45. doi: 10.1016/j.biocontrol.2017.01.018. - DOI
-
- Bamisile B.S., Dash C.K., Akutse K.S., Keppanan R., Afolabi O.G., Hussain M., Qasim M., Wang L. Prospects of endophytic fungal entomopathogens as biocontrol and plant growth promoting agents: An insight on how artificial inoculation methods affect endophytic colonization of host plants. Microbiol. Res. 2018;217:34–50. doi: 10.1016/j.micres.2018.08.016. - DOI - PubMed
-
- Petrini O. Fungal Endophytes of Tree Leaves. In: Andrews J.H., Hirano S.S., editors. Microbial Ecology of Leaves. Springer; New York, NY, USA: 1991. pp. 179–197. - DOI
LinkOut - more resources
Full Text Sources

