Attenuation of Pseudomonas aeruginosa Virulence by Pomegranate Peel Extract
- PMID: 36557753
- PMCID: PMC9784079
- DOI: 10.3390/microorganisms10122500
Attenuation of Pseudomonas aeruginosa Virulence by Pomegranate Peel Extract
Abstract
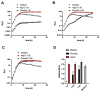
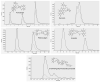
Pseudomonas aeruginosa is an opportunistic pathogen often responsible for biofilm-associated infections. The high adhesion of bacterial cells onto biotic/abiotic surfaces is followed by production of an extracellular polysaccharidic matrix and formation of a sessile community (the biofilm) by the release of specific quorum-sensing molecules, named autoinducers (AI). When the concentrations of AI reach a threshold level, they induce the expression of many virulence genes, including those involved in biofilm formation, motility, pyoverdine and pyocyanin release. P. aeruginosa embedded into biofilm becomes resistant to both conventional drugs and the host's immune response. Accordingly, biofilm-associated infections are a major clinical problem underlining the need for new antimicrobial therapies. In this study, we evaluated the effects of pomegranate peel extract (PomeGr) in vitro on P. aeruginosa growth and biofilm formation; moreover, the release of four AI was assessed. The phenolic profile of PomeGr, exposed or not to bacteria, was determined by high-performance liquid chromatography coupled to electrospray ionization mass spectrometry (HPLC-ESI-MS) analysis. We found that bacterial growth, biofilm production and AI release were impaired upon PomeGr treatment. In addition, the PomeGr phenolic content was also markedly hampered following incubation with bacterial cells. In particular, punicalagin, punicalin, pedunculagin, granatin, di-(HHDP-galloyl-hexoside) pentoside and their isomers were highly consumed. Overall, these results provide novel insights on the ability of PomeGr to attenuate P. aeruginosa virulence; moreover, the AI impairment and the observed consumption of specific phenolic compounds may offer new tools in designing innovative therapeutic approaches against bacterial infections.
Keywords: Pseudomonas aeruginosa; anti-biofilm; autoinducers; phenolic compounds; pomegranate; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Pomegranate Extract Affects Fungal Biofilm Production: Consumption of Phenolic Compounds and Alteration of Fungal Autoinducers Release.Int J Environ Res Public Health. 2022 Oct 29;19(21):14146. doi: 10.3390/ijerph192114146. Int J Environ Res Public Health. 2022. PMID: 36361021 Free PMC article.
-
Inhibition of biofilm and quorum sensing-regulated virulence factors in Pseudomonas aeruginosa by Cuphea carthagenensis (Jacq.) J. F. Macbr. Leaf extract: An in vitro study.J Ethnopharmacol. 2021 Apr 6;269:113699. doi: 10.1016/j.jep.2020.113699. Epub 2020 Dec 17. J Ethnopharmacol. 2021. PMID: 33340600
-
Musa acuminata and its bioactive metabolite 5-Hydroxymethylfurfural mitigates quorum sensing (las and rhl) mediated biofilm and virulence production of nosocomial pathogen Pseudomonas aeruginosa in vitro.J Ethnopharmacol. 2020 Jan 10;246:112242. doi: 10.1016/j.jep.2019.112242. Epub 2019 Sep 15. J Ethnopharmacol. 2020. PMID: 31533077
-
Sesamin and sesamolin rescues Caenorhabditis elegans from Pseudomonas aeruginosa infection through the attenuation of quorum sensing regulated virulence factors.Microb Pathog. 2021 Jun;155:104912. doi: 10.1016/j.micpath.2021.104912. Epub 2021 Apr 28. Microb Pathog. 2021. PMID: 33932548 Review.
-
Recent perspectives on the molecular basis of biofilm formation by Pseudomonas aeruginosa and approaches for treatment and biofilm dispersal.Folia Microbiol (Praha). 2018 Jul;63(4):413-432. doi: 10.1007/s12223-018-0585-4. Epub 2018 Jan 19. Folia Microbiol (Praha). 2018. PMID: 29352409 Review.
Cited by
-
Antibacterial Properties of the Antimicrobial Peptide Gallic Acid-Polyphemusin I (GAPI).Antibiotics (Basel). 2023 Aug 22;12(9):1350. doi: 10.3390/antibiotics12091350. Antibiotics (Basel). 2023. PMID: 37760647 Free PMC article.
-
Antimicrobial efficacy of Punica granatum Lythraceae peel extract against pathogens belonging to the ESKAPE group.Front Microbiol. 2024 Apr 22;15:1383027. doi: 10.3389/fmicb.2024.1383027. eCollection 2024. Front Microbiol. 2024. PMID: 38711969 Free PMC article.
-
Effects of pomegranate extract on preventing dental caries: a systematic review.Front Oral Health. 2025 May 27;6:1484364. doi: 10.3389/froh.2025.1484364. eCollection 2025. Front Oral Health. 2025. PMID: 40496699 Free PMC article.
References
LinkOut - more resources
Full Text Sources

