Living and Regenerative Material Encapsulating Self-Assembled Shewanella oneidensis-CdS Hybrids for Photocatalytic Biodegradation of Organic Dyes
- PMID: 36557754
- PMCID: PMC9781410
- DOI: 10.3390/microorganisms10122501
Living and Regenerative Material Encapsulating Self-Assembled Shewanella oneidensis-CdS Hybrids for Photocatalytic Biodegradation of Organic Dyes
Abstract
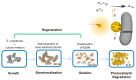
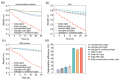
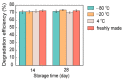
Reductive biodegradation by microorganisms has been widely explored for detoxifying recalcitrant contaminants; however, the biodegradation capacity of microbes is limited by the energy level of the released electrons. Here, we developed a method to self-assemble Shewanella oneidensis-CdS nanoparticle hybrids with significantly improved reductive biodegradation capacity and constructed a living material by encapsulating the hybrids in hydrogels. The material confines the nano-bacteria hybrids and protects them from environmental stress, thus improving their recyclability and long-term stability (degradation capacity unhindered after 4 weeks). The developed living materials exhibited efficient photocatalytic biodegradation of various organic dyes including azo and nitroso dyes. This study highlights the feasibility and benefits of constructing self-assembled nano-bacteria hybrids for bioremediation and sets the stage for the development of novel living materials from nano-bacteria hybrids.
Keywords: Shewanella oneidensis; biodegradation; hydrogel; living material; nano-bacteria hybrid.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources

