Natural Infections of Potato Plants Grown from Minitubers with Blackleg-Causing Soft Rot Pectobacteriaceae
- PMID: 36557757
- PMCID: PMC9787864
- DOI: 10.3390/microorganisms10122504
Natural Infections of Potato Plants Grown from Minitubers with Blackleg-Causing Soft Rot Pectobacteriaceae
Abstract
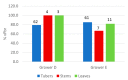
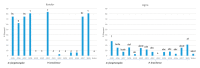
Information on the infection incidence of blackleg-causing soft rot Pectobacteriaceae (BL-SRP) in potato crops grown from minitubers (PB1-crop) and the distribution of BL-SRP in individual plants was collected during a two-year survey conducted at five potato growers located in the Netherlands. In the last weeks before haulm destruction, leaves, stems, and tubers of 100 or 200 plants were analyzed separately for the presence of Pectobacterium parmentieri, P. brasiliense, P. atrosepticum, and Dickeya spp. Extracted plant parts enriched for BL-SRP were analyzed with TaqMan assays specific for the detection of blackleg-causing BL-SRP. In 2019, low incidences of P. parmentieri (1-6%) in leaves were found at four growing sites. At one farm, reactions were detected in TaqMan assays for D. zeae and D. chrysanthemi in leaves. In 2020, the crops of two growers were largely free from BL-SRP. At one farm, a high infection incidence (21%) was found for D. fangzhongdai in tubers. The isolated pathogen was able to cause potato blackleg. At two other farms, high infection incidences in tubers were found with P. brasiliense (35-39%) and P. parmentieri (12-19%), whereas the incidence of P. brasiliense in leaves was also high (8%). In conclusion, high infection incidences with BL-SRP in potatoes can be found in a PB1 crop at the end of the growing season. Infections in individual plants were found either in tubers or in leaves. The potential sources of initial infection are discussed.
Keywords: Dickeya; Pectobacterium; TaqMan; airborne infection; gapA sequencing; infection source; initial infection; soilborne infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Multilocus sequence and phenotypic analysis of Pectobacterium and Dickeya type strains for identification of soft rot Pectobacteriaceae from symptomatic potato stems and tubers in Pennsylvania.Syst Appl Microbiol. 2024 Jan;47(1):126476. doi: 10.1016/j.syapm.2023.126476. Epub 2023 Nov 26. Syst Appl Microbiol. 2024. PMID: 38113702
-
First Report of Pectobacterium carotovorum subsp. brasiliense Causing Soft Rot and Blackleg of Potatoes in Kenya.Plant Dis. 2014 May;98(5):684. doi: 10.1094/PDIS-09-13-0988-PDN. Plant Dis. 2014. PMID: 30708532
-
Identification of Pectobacterium versatile Causing Blackleg of Potato in New York State.Plant Dis. 2021 Sep;105(9):2585-2594. doi: 10.1094/PDIS-09-20-2089-RE. Epub 2021 Oct 24. Plant Dis. 2021. PMID: 33404272
-
Looking for Resistance to Soft Rot Disease of Potatoes Facing Environmental Hypoxia.Int J Mol Sci. 2024 Mar 28;25(7):3757. doi: 10.3390/ijms25073757. Int J Mol Sci. 2024. PMID: 38612570 Free PMC article. Review.
-
Pectobacterium brasiliense: Genomics, Host Range and Disease Management.Microorganisms. 2021 Jan 5;9(1):106. doi: 10.3390/microorganisms9010106. Microorganisms. 2021. PMID: 33466309 Free PMC article. Review.
Cited by
-
Role of Volatile Organic Compounds Produced by Kosakonia cowanii Cp1 during Competitive Colonization Interaction against Pectobacterium aroidearum SM2.Microorganisms. 2024 May 3;12(5):930. doi: 10.3390/microorganisms12050930. Microorganisms. 2024. PMID: 38792761 Free PMC article.
-
Retrospective survey of Dickeya fangzhongdai using a novel validated real-time PCR assay.Front Microbiol. 2024 Feb 13;14:1249955. doi: 10.3389/fmicb.2023.1249955. eCollection 2023. Front Microbiol. 2024. PMID: 38414710 Free PMC article.
-
Field Use of Protective Bacteriophages against Pectinolytic Bacteria of Potato.Microorganisms. 2023 Feb 28;11(3):620. doi: 10.3390/microorganisms11030620. Microorganisms. 2023. PMID: 36985194 Free PMC article.
References
-
- Ma B., Hibbing M.E., Kim H.S., Reedy R.M., Yedidia I., Breuer J., Breuer J., Glasner J.D., Perna N.T., Kelman A., et al. Host range and molecular phylogenies of the soft rot enterobacterial genera Pectobacterium and Dickeya. Phytopathology. 2007;97:1150–1163. doi: 10.1094/PHYTO-97-9-1150. - DOI - PubMed
-
- Toth I.K., Barny M.-A., Brurberg M.B., Condemine G., Czajkowski R., Elphinstone J.G., Helias V., Johnson S.B., Moleleki L.N., Pirhonen M., et al. Plant Diseases Caused by Dickeya and Pectobacterium Species. Springer; Berlin/Heidelberg, Germany: 2021. Pectobacterium and Dickeya: Environment to Disease Development; pp. 39–84.
-
- Van der Wolf J.M., Acuña I., De Boer S.H., Brurberg M.B., Cahill G., Charkowski A.O., Coutinho T., Davey T., Dees M.W., Degefu Y. Plant Diseases Caused by Dickeya and Pectobacterium Species. Springer; Berlin/Heidelberg, Germany: 2021. Diseases Caused by Pectobacterium and Dickeya species around the world. Chapter 7; pp. 215–261.
-
- Czajkowski R., Perombelon M.C.M., van Veen J.A., van der Wolf J.M. Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: A review. Plant Pathol. 2011;60:999–1013. doi: 10.1111/j.1365-3059.2011.02470.x. - DOI
-
- Van der Wolf J.M., De Boer S.H., Czajkowski R., Cahill G., Van Gijsegem F., Davey T., Dupuis B., Ellicott J., Jafra S., Kooman M. Plant Diseases Caused by Dickeya and Pectobacterium Species. Springer; Berlin/Heidelberg, Germany: 2021. Management of diseases caused by Pectobacterium and Dickeya species. Chapter 6; pp. 175–214.
LinkOut - more resources
Full Text Sources
Miscellaneous

