Opuntia dillenii Haw. Polysaccharide Promotes Cholesterol Efflux in THP-1-Derived Foam Cells via the PPARγ-LXRα Signaling Pathway
- PMID: 36557773
- PMCID: PMC9781717
- DOI: 10.3390/molecules27248639
Opuntia dillenii Haw. Polysaccharide Promotes Cholesterol Efflux in THP-1-Derived Foam Cells via the PPARγ-LXRα Signaling Pathway
Abstract
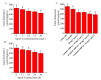
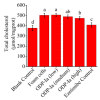
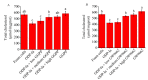
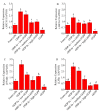
There is increasing evidence supporting a role for enhanced macrophage cholesterol efflux in ameliorating atherosclerosis. Opuntia dillenii Haw. polysaccharide (ODP-Ia), the most important functional component obtained from Opuntia dillenii Haw. stem, has anti-atherosclerosis effects. Therefore, we propose that ODP-Ia could promote cholesterol efflux via the PPARγ-LXRα signaling pathway. In this study, THP-1 foam cells derived from macrophages were treated with different concentrations of ODP-Ia, GGPP (antagonist of LXRα) and GW9662 (antagonist of PPARγ), with or without 15 nmol ODP-Ia. The total cholesterol content in the cells was measured. The mRNA of ABCA1, ABCG1, PPARγ, LXRα and their protein levels in the foam cells were detected by RT−PCR and Western blot, respectively. The results showed that ODP-Ia plays a role in significantly promoting cholesterol efflux (p < 0.05) by upregulating the expression of ABCA1, ABCG1, SR-BI, PPARγ, PPARα and LXRα. Meanwhile, PPARγ and LXRα antagonists dramatically interfered the cholesterol efflux mediated by ODP-Ia (p < 0.05) and dramatically inhibited the upregulating effect of ODP-Ia on the expression of PPARγ, LXRα, ABCA1 and ABCG1 at both protein and mRNA levels (p < 0.05). In conclusion, ODP-Ia promotes cholesterol efflux in the foam cells through activating the PPARγ-LXRα signaling pathway. This bioactivity suggested that ODP-Ia may be of benefit in treating atherosclerosis.
Keywords: ABCA1; Opuntia dillenii Haw. polysaccharide; PPARγ-LXRα; THP-1-derived foam cells; cholesterol efflux.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

