Electrochemical Exfoliation of Graphite to Graphene-Based Nanomaterials
- PMID: 36557776
- PMCID: PMC9783006
- DOI: 10.3390/molecules27248643
Electrochemical Exfoliation of Graphite to Graphene-Based Nanomaterials
Abstract
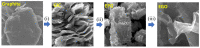
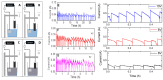
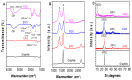
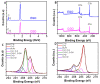
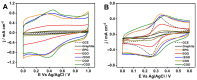
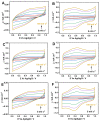
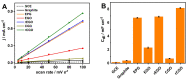
Here, we report on a new automated electrochemical process for the production of graphene oxide (GO) from graphite though electrochemical exfoliation. The effects of the electrolyte and applied voltage were investigated and optimized. The morphology, structure and composition of the electrochemically exfoliated GO (EGO) were probed by scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), energy dispersive X-ray (EDX) spectroscopy, X-ray photoelectron spectroscopy (XPS), FTIR spectroscopy and Raman spectroscopy. Important metrics such as the oxygen content (25.3 at.%), defect density (ID/IG = 0.85) and number of layers of the formed EGO were determined. The EGO was also compared with the GO prepared using the traditional chemical method, demonstrating the effectiveness of the automated electrochemical process. The electrochemical properties of the EGO, CGO and other carbon-based materials were further investigated and compared. The automated electrochemical exfoliation of natural graphite powder demonstrated in the present study does not require any binders; it is facile, cost-effective and easy to scale up for a large-scale production of graphene-based nanomaterials for various applications.
Keywords: electrochemical exfoliation; energy storage; expanded graphite; graphene oxide; graphite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Smith A.T., LaChance A.M., Zeng S., Liu B., Sun L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019;1:31–47. doi: 10.1016/j.nanoms.2019.02.004. - DOI
-
- Thiruppathi A.R., Sidhureddy B., Salverda M., Wood P.C., Chen A. Novel three-dimensional N-doped interconnected reduced graphene oxide with superb capacitance for energy storage. J. Electroanal. Chem. 2020;875:113911. doi: 10.1016/j.jelechem.2020.113911. - DOI
-
- Mantovani S., Khaliha S., Favaretto L., Bettini C., Bianchi A., Kovtun A., Zambianchi M., Gazzano M., Casentini B., Palermo V., et al. Scalable synthesis and purification of functionalized graphene nanosheets for water remediation. Chem. Commun. 2021;57:3765–3768. doi: 10.1039/D1CC00704A. - DOI - PubMed
-
- Thiruppathi A.R., Sidhureddy B., Keeler W., Chen A. Facile one-pot synthesis of fluorinated graphene oxide for electrochemical sensing of heavy metal ions. Electrochem. Commun. 2017;76:42–46. doi: 10.1016/j.elecom.2017.01.015. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

