Efficient Degradation of Congo Red in Water by UV-Vis Driven CoMoO4/PDS Photo-Fenton System
- PMID: 36557777
- PMCID: PMC9784357
- DOI: 10.3390/molecules27248642
Efficient Degradation of Congo Red in Water by UV-Vis Driven CoMoO4/PDS Photo-Fenton System
Abstract
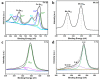
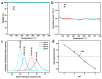
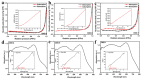
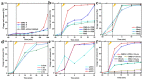
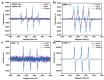
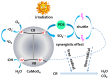
In order to improve the catalytic activity of cobalt molybdate (CoMoO4), a PDS-activated and UV-vis assisted system was constructed. CoMoO4 was prepared by coprecipitation and calcination, and characterized by XRD, FTIR, Raman, SEM, TEM, XPS, TGA Zeta potential, BET, and UV-Vis DRS. The results showed that the morphology of the CoMoO4 nanolumps consisted of stacked nanosheets. XRD indicated the monoclinic structures with C2/m (C32h, #12) space group, which belong to α-CoMoO4, and both Co2+ and Mo6+ ions occupy distorted octahedral sites. The pH of the isoelectric point (pHIEP) of CMO-8 at pH = 4.88 and the band gap of CoMoO4 was 1.92 eV. The catalytic activity of CoMoO4 was evaluated by photo-Fenton degradation of Congo red (CR). The catalytic performance was affected by calcination temperature, catalyst dosage, PDS dosage, and pH. Under the best conditions (0.8 g/L CMO-8, PDS 1 mL), the degradation efficiency of CR was 96.972%. The excellent catalytic activity of CoMoO4 was attributed to the synergistic effect of photo catalysis and CoMoO4-activated PDS degradation. The capture experiments and the ESR showed that superoxide radical (·O2-), singlet oxygen (1O2), hole (h+), sulfate (SO4-·), and hydroxyl (·OH-) were the main free radicals leading to the degradation of CR. The results can provide valuable information and support for the design and application of high-efficiency transition metal oxide catalysts.
Keywords: CoMoO4; Congo red; PDS; photo-Fenton.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
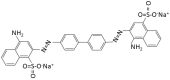



Similar articles
-
Sol-Gel Synthesis and Characterizations of CoMoO4 Nanoparticles: An Efficient Photocatalytic Degradation of 4-Chlorophenol.J Nanosci Nanotechnol. 2016 Mar;16(3):2960-6. doi: 10.1166/jnn.2016.10761. J Nanosci Nanotechnol. 2016. PMID: 27455742
-
Metronidazole degradation mechanism by sono-photo-Fenton processes using a spinel ferrite cobalt on activated carbon catalyst.Chemosphere. 2024 Jun;358:142102. doi: 10.1016/j.chemosphere.2024.142102. Epub 2024 Apr 25. Chemosphere. 2024. PMID: 38677611
-
Visible-light photo-Fenton oxidation of phenol with rGO-α-FeOOH supported on Al-doped mesoporous silica (MCM-41) at neutral pH: Performance and optimization of the catalyst.Chemosphere. 2017 Sep;182:468-476. doi: 10.1016/j.chemosphere.2017.05.037. Epub 2017 May 7. Chemosphere. 2017. PMID: 28521161
-
Magnetic hierarchical flower-like Fe3O4@ZIF-67/CuNiMn-LDH catalyst with enhanced redox cycle for Fenton-like degradation of Congo red: optimization and mechanism.Environ Sci Pollut Res Int. 2023 Jun;30(30):75332-75348. doi: 10.1007/s11356-023-27430-2. Epub 2023 May 23. Environ Sci Pollut Res Int. 2023. PMID: 37219772 Free PMC article.
-
Recent advances in cobalt-activated sulfate radical-based advanced oxidation processes for water remediation: A review.Sci Total Environ. 2021 May 20;770:145311. doi: 10.1016/j.scitotenv.2021.145311. Epub 2021 Jan 21. Sci Total Environ. 2021. PMID: 33736411 Review.
Cited by
-
Enhanced Redox Cycle of Rod-Shaped MIL-88A/SnFe2O4@MXene Sheets for Fenton-like Degradation of Congo Red: Optimization and Mechanism.Nanomaterials (Basel). 2023 Dec 24;14(1):54. doi: 10.3390/nano14010054. Nanomaterials (Basel). 2023. PMID: 38202509 Free PMC article.
-
Tailoring Vanadium-Based Magnetic Catalyst by In Situ Encapsulation of Tungsten Disulfide and Applications in Abatement of Multiple Pollutants.ACS Omega. 2023 Dec 12;8(51):48966-48974. doi: 10.1021/acsomega.3c06580. eCollection 2023 Dec 26. ACS Omega. 2023. PMID: 38162758 Free PMC article.
References
-
- Haounati R., Alakhras F., Ouachtak H., Saleh T., Al-Mazaideh G., Alhajri E., Jada A., Hafid N., Addi A. Synthesized of Zeolite@Ag2O Nanocomposite as Superb Stability Photocatalysis Toward Hazardous Rhodamine B Dye from Water. Arab. J. Sci. Eng. 2022;2022:1–11. doi: 10.1007/s13369-022-06899-y. - DOI
-
- Haounati R., El Guerdaoui A., Ouachtak H., El Haouti R., Bouddouch A., Hafid N., Bakiz B., Santos D.M.F., Taha M.L., Jada A., et al. Design of direct Z-scheme superb magnetic nanocomposite photocatalyst Fe3O4/Ag3PO4@Sep for hazardous dye degradation. Sep. Purif. Technol. 2021;277:119399.
-
- Das T., Ganguly S., Bhawal1 P., Mondal1 S., Das N. A facile green synthesis of silver nanoparticle-decorated hydroxyapatite for efficient catalytic activity towards 4-nitrophenol reduction. Res. Chem. Intermed. 2018;44:1189–1208. doi: 10.1007/s11164-017-3161-7. - DOI
-
- Das T., Remanan S., Ghosh S., Ghosh S., Das N. Efficient synthesis of catalytic active silver nanoparticles illuminated cerium oxide nanotube: A mussel inspired approach. Environ. Nanotechnol. Monit. Manag. 2021;15:100411. doi: 10.1016/j.enmm.2020.100411. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

