Anti-Mycobacterial N-(2-Arylethyl)quinolin-3-amines Inspired by Marine Sponge-Derived Alkaloid
- PMID: 36557834
- PMCID: PMC9781020
- DOI: 10.3390/molecules27248701
Anti-Mycobacterial N-(2-Arylethyl)quinolin-3-amines Inspired by Marine Sponge-Derived Alkaloid
Abstract
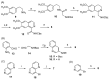
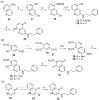
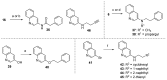
The synthesis and evaluation of simplified analogs of marine sponge-derived alkaloid 3-(phenethylamino)demethyl(oxy)aaptamine were performed to develop novel anti-mycobacterial substances. Ring truncation of the tricyclic benzo[de][1,6]-naphthyridine skeleton effectively weakened the cytotoxicity of the natural product, and the resulting AC-ring analog exhibited good anti-mycobacterial activity. A structure-activity relationship (SAR) study, synthesizing and evaluating some analogs, demonstrated the specificity and importance of the N-(2-arylethyl)quinolin-3-amine skeleton as a promising scaffold for anti-mycobacterial lead compounds.
Keywords: aaptamine; anti-mycobacterial; marine natural product; truncated analog.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Four new aaptamine alkaloids from marine sponge Aaptos aaptos.Nat Prod Res. 2022 Oct;36(19):5022-5031. doi: 10.1080/14786419.2021.1917572. Epub 2021 Apr 28. Nat Prod Res. 2022. PMID: 33908314
-
Suberitine A-D, four new cytotoxic dimeric aaptamine alkaloids from the marine sponge Aaptos suberitoides.Org Lett. 2012 Apr 20;14(8):1994-7. doi: 10.1021/ol3004589. Epub 2012 Apr 3. Org Lett. 2012. PMID: 22472093
-
Review on marine sponge alkaloid, aaptamine: A potential antibacterial and anticancer drug.Chem Biol Drug Des. 2022 Jan;99(1):103-110. doi: 10.1111/cbdd.13932. Epub 2021 Aug 22. Chem Biol Drug Des. 2022. PMID: 34331335 Review.
-
Study of the Structure-Activity Relationship of an Anti-Dormant Mycobacterial Substance 3-(Phenethylamino)Demethyl(oxy)aaptamine to Create a Probe Molecule for Detecting Its Target Protein.Mar Drugs. 2022 Jan 24;20(2):98. doi: 10.3390/md20020098. Mar Drugs. 2022. PMID: 35200628 Free PMC article.
-
Novel antitumor agents: marine sponge alkaloids, their synthetic analogs and derivatives.Mini Rev Med Chem. 2005 Mar;5(3):319-36. doi: 10.2174/1389557053175362. Mini Rev Med Chem. 2005. PMID: 15777266 Review.
References
-
- World Health Organization Global Tuberculosis Report 2021. [(accessed on 10 November 2021)]. Available online: https://www.who.int/publications/i/item/9789240037021.
MeSH terms
Substances
Grants and funding
- JP22ama121054/Research Support Project for Life Science and Drug Discovery (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from AMED
- no. B21-0051/the Research Promotion Program for Acquiring KAKENHI from Ritsumeikan University
- no. 18K05363/Grant-in-Aid for Scientific Research C from the Japan Society for the Promotion of Science
- no. 21H02069/Grant-in-Aid for Scientific Research B from JSPS
LinkOut - more resources
Full Text Sources
Miscellaneous

