ML216-Induced BLM Helicase Inhibition Sensitizes PCa Cells to the DNA-Crosslinking Agent Cisplatin
- PMID: 36557923
- PMCID: PMC9788632
- DOI: 10.3390/molecules27248790
ML216-Induced BLM Helicase Inhibition Sensitizes PCa Cells to the DNA-Crosslinking Agent Cisplatin
Abstract
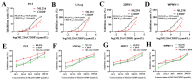
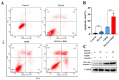
Using standard DNA-damaging medicines with DNA repair inhibitors is a promising anticancer tool to achieve better therapeutic responses and reduce therapy-related side effects. Cell viability assay, neutral comet assay, western blotting (WB), and cell cycle and apoptosis analysis were used to determine the synergistic effect and mechanism of ML216, a Bloom syndrome protein (BLM) helicase inhibitor, and cisplatin (CDDP), a DNA-crosslinking agent, in PCa cells. Based on the online database research, our findings revealed that BLM was substantially expressed in PCa, which is associated with a bad prognosis for PCa patients. The combination of ML216 and CDDP improved the antiproliferative properties of three PCa cell lines. As indicated by the increased production of γH2AX and caspase-3 cleavage, ML216 significantly reduced the DNA damage-induced high expression of BLM, making PC3 more susceptible to apoptosis and DNA damage caused by CDDP. Furthermore, the combination of ML216 and CDDP increased p-Chk1 and p-Chk2 expression. The DNA damage may have triggered the ATR-Chk1 and ATM-Chk2 pathways simultaneously. Our results demonstrated that ML216 and CDDP combination therapy exhibited synergistic effects, and combination chemotherapy could be a novel anticancer tactic.
Keywords: BLM inhibitor; CDDP; ML216; combined therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Asangani I.A., Wilder-Romans K., Dommeti V.L., Krishnamurthy P.M., Apel I.J., Escara-Wilke J., Plymate S.R., Navone N.M., Wang S., Feng F.Y., et al. BET Bromodomain Inhibitors Enhance Efficacy and Disrupt Resistance to AR Antagonists in the Treatment of Prostate Cancer. Mol Cancer Res. 2016;14:324–331. doi: 10.1158/1541-7786.MCR-15-0472. - DOI - PMC - PubMed
-
- Madan R.A., Karzai F.H., Ning Y.M., Adesunloye B.A., Huang X., Harold N., Couvillon A., Chun G., Cordes L., Sissung T., et al. Phase II trial of docetaxel, bevacizumab, lenalidomide and prednisone in patients with metastatic castration-resistant prostate cancer. BJU Int. 2016;118:590–597. doi: 10.1111/bju.13412. - DOI - PMC - PubMed
-
- Gravis G., Boher J.M., Chen Y.H., Liu G., Fizazi K., Carducci M.A., Oudard S., Joly F., Jarrard D.M., Soulie M., et al. Burden of Metastatic Castrate Naive Prostate Cancer Patients, to Identify Men More Likely to Benefit from Early Docetaxel: Further Analyses of CHAARTED and GETUG-AFU15 Studies. Eur. Urol. 2018;73:847–855. doi: 10.1016/j.eururo.2018.02.001. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

