Production of [11C]Carbon Labelled Flumazenil and L-Deprenyl Using the iMiDEV™ Automated Microfluidic Radiosynthesizer
- PMID: 36557975
- PMCID: PMC9788284
- DOI: 10.3390/molecules27248843
Production of [11C]Carbon Labelled Flumazenil and L-Deprenyl Using the iMiDEV™ Automated Microfluidic Radiosynthesizer
Abstract
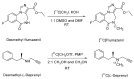
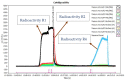
In the last decade, microfluidic techniques have been explored in radiochemistry, and some of them have been implemented in preclinical production. However, these are not suitable and reliable for preparing different types of radiotracers or dose-on-demand production. A fully automated iMiDEV™ microfluidic radiosynthesizer has been introduced and this study is aimed at using of the iMiDEV™ radiosynthesizer with a microfluidic cassette to produce [11C]flumazenil and [11C]L-deprenyl. These two are known PET radioligands for benzodiazepine receptors and monoamine oxidase-B (MAO-B), respectively. Methods were successfully developed to produce [11C]flumazenil and [11C]L-deprenyl using [11C]methyl iodide and [11C]methyl triflate, respectively. The final products 1644 ± 504 MBq (n = 7) and 533 ± 20 MBq (n = 3) of [11C]flumazenil and [11C]L-deprenyl were produced with radiochemical purities were over 98% and the molar activity for [11C]flumazenil and [11C]L-deprenyl was 1912 ± 552 GBq/µmol, and 1463 ± 439 GBq/µmol, respectively, at the end of synthesis. All the QC tests complied with the European Pharmacopeia. Different parameters, such as solvents, bases, methylating agents, precursor concentration, and different batches of cassettes, were explored to increase the radiochemical yield. Synthesis methods were developed using 3-5 times less precursor than conventional methods. The fully automated iMiDEV™ microfluidic radiosynthesizer was successfully applied to prepare [11C]flumazenil and [11C]L-deprenyl.
Keywords: PET radiotracers; [11C]L-deprenyl; [11C]flumazenil; dose-on-demand (DOD); iMiDEV™; microfluidic cassette; microfluidics; radiosynthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Basu S., Alavi A. Unparalleled contribution of 18F-FDG PET to medicine over 3 decades. [(accessed on 7 October 2022)];J. Nucl. Med. 2008 49:17N–37N. Available online: http://www.ncbi.nlm.nih.gov/pubmed/18832112. - PubMed
-
- Morrish P.K., Rakshi J.S., Bailey D.L., Sawle G.V., Brooks D.J. Measuring the rate of progression and estimating the preclinical period of parkinson’s disease with [18f]dopa pet. [(accessed on 7 October 2022)];J. Neurol. Neurosurg. Psychiatry. 1998 64:314–319. doi: 10.1136/jnnp.64.3.314. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9527140. - DOI - PMC - PubMed
-
- Tolboom N., Yaqub M., van der Flier W.M., Boellaard R., Luurtsema G., Windhorst A.D., Barkhof F., Scheltens P., Lammertsma A.A., van Berckel B.N. Detection of Alzheimer Pathology In Vivo Using Both 11C-PIB and 18F-FDDNP PET. J. Nucl. Med. 2009;50:191–197. doi: 10.2967/jnumed.108.056499. - DOI - PubMed
-
- Halldin C., Gulyás B., Langer O., Farde L. Brain radioligands—State of the art and new trends. Q. J. Nucl. Med. 2001;45:139–152. - PubMed
-
- Guadagno J.V., Warburton E.A., Jones P.S., Day D.J., Aigbirhio F.I., Fryer T.D., Harding S., Price C., Green H.A., Barret O., et al. How affected is oxygen metabolism in DWI lesions?: A combined acute stroke PET-MR study. Neurology. 2006;67:824–829. doi: 10.1212/01.wnl.0000233984.66907.db. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

