Exploring the Dynamical Nature of Intermolecular Hydrogen Bonds in Benzamide, Quinoline and Benzoic Acid Derivatives
- PMID: 36557978
- PMCID: PMC9783803
- DOI: 10.3390/molecules27248847
Exploring the Dynamical Nature of Intermolecular Hydrogen Bonds in Benzamide, Quinoline and Benzoic Acid Derivatives
Abstract
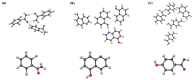
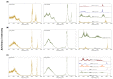
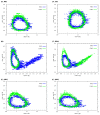
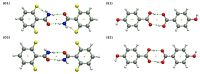
The hydrogen bonds properties of 2,6-difluorobenzamide, 5-hydroxyquinoline and 4-hydroxybenzoic acid were investigated by Car-Parrinello and path integral molecular dynamics (CPMD and PIMD), respectively. The computations were carried out in vacuo and in the crystalline phase. The studied complexes possess diverse networks of intermolecular hydrogen bonds (N-H…O, O-H…N and O-H…O). The time evolution of hydrogen bridges gave a deeper insight into bonds dynamics, showing that bridged protons are mostly localized on the donor side; however, the proton transfer phenomenon was registered as well. The vibrational features associated with O-H and N-H stretching were analyzed on the basis of the Fourier transform of the atomic velocity autocorrelation function. The spectroscopic effects of hydrogen bond formation were studied. The PIMD revealed quantum effects influencing the hydrogen bridges providing more accurate free energy sampling. It was found that the N…O or O…O interatomic distances decreased (reducing the length of the hydrogen bridge), while the O-H or N-H covalent bond was elongated, which led to the increase in the proton sharing. Furthermore, Quantum Theory of Atoms in Molecules (QTAIM) was used to give insight into electronic structure parameters. Finally, Symmetry-Adapted Perturbation Theory (SAPT) was employed to estimate the energy contributions to the interaction energy of the selected dimers.
Keywords: CPMD; PIMD; QTAIM; SAPT; hydrogen bond; non-covalent interactions; spectroscopic signatures.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Revealing Intra- and Intermolecular Interactions Determining Physico-Chemical Features of Selected Quinolone Carboxylic Acid Derivatives.Molecules. 2022 Apr 1;27(7):2299. doi: 10.3390/molecules27072299. Molecules. 2022. PMID: 35408698 Free PMC article.
-
Unraveling the Nature of Hydrogen Bonds of "Proton Sponges" Based on Car-Parrinello and Metadynamics Approaches.Int J Mol Sci. 2023 Jan 12;24(2):1542. doi: 10.3390/ijms24021542. Int J Mol Sci. 2023. PMID: 36675059 Free PMC article.
-
Sensitivity of Intra- and Intermolecular Interactions of Benzo[h]quinoline from Car-Parrinello Molecular Dynamics and Electronic Structure Inspection.Int J Mol Sci. 2021 May 14;22(10):5220. doi: 10.3390/ijms22105220. Int J Mol Sci. 2021. PMID: 34069244 Free PMC article.
-
Crystalline Peroxosolvates: Nature of the Coformer, Hydrogen-Bonded Networks and Clusters, Intermolecular Interactions.Molecules. 2020 Dec 23;26(1):26. doi: 10.3390/molecules26010026. Molecules. 2020. PMID: 33374602 Free PMC article. Review.
-
The Basics of Covalent Bonding in Terms of Energy and Dynamics.Molecules. 2020 Jun 8;25(11):2667. doi: 10.3390/molecules25112667. Molecules. 2020. PMID: 32521828 Free PMC article. Review.
Cited by
-
Static and Dynamical Quantum Studies of CX3-AlX2 and CSiX3-BX2 (X = F, Cl, Br) Complexes with Hydrocyanic Acid: Unusual Behavior of Strong π-Hole at Triel Center.Int J Mol Sci. 2023 Apr 26;24(9):7881. doi: 10.3390/ijms24097881. Int J Mol Sci. 2023. PMID: 37175586 Free PMC article.
-
Hydrogen Bonds.Molecules. 2023 Feb 8;28(4):1616. doi: 10.3390/molecules28041616. Molecules. 2023. PMID: 36838604 Free PMC article.
References
-
- Pauling L. The Nature of the Chemical Bond and the Structure of Molecules and Crystals; An Introduction to Modern Structural Chemistry. 3rd ed. Cornell University Press; Ithaca, NY, USA: 1960.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

