The Effects of Iron on In Silico Simulated Abiotic Reaction Networks
- PMID: 36558002
- PMCID: PMC9787479
- DOI: 10.3390/molecules27248870
The Effects of Iron on In Silico Simulated Abiotic Reaction Networks
Abstract
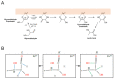
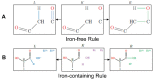
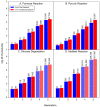
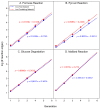
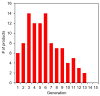
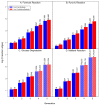
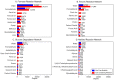
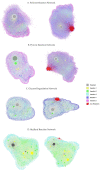
Iron is one of the most abundant elements in the Universe and Earth's surfaces, and undergoes a redox change of approximately 0.77 mV in changing between its +2 and +3 states. Many contemporary terrestrial organisms are deeply connected to inorganic geochemistry via exploitation of this redox change, and iron redox reactions and catalysis are known to cause significant changes in the course of complex abiotic reactions. These observations point to the question of whether iron may have steered prebiotic chemistry during the emergence of life. Using kinetically naive in silico reaction modeling we explored the potential effects of iron ions on complex reaction networks of prebiotic interest, namely the formose reaction, the complexifying degradation reaction of pyruvic acid in water, glucose degradation, and the Maillard reaction. We find that iron ions produce significant changes in the connectivity of various known diversity-generating reaction networks of proposed prebiotic significance, generally significantly diversifying novel molecular products by ~20%, but also adding the potential for kinetic effects that could allow iron to steer prebiotic chemistry in marked ways.
Keywords: Maillard reaction; chemical reaction networks; combinatorial chemistry; formose reaction; glucose; iron chemistry; iron-sulfur world; origins of life; prebiotic chemistry; pyruvic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Dhungana S., Crumbliss A.L. Coordination Chemistry and Redox Processes in Siderophore-Mediated Iron Transport. Geomicrobiol. J. 2005;22:87–98. doi: 10.1080/01490450590945870. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

