Photodynamic Inactivation of Bacteria and Biofilms with Benzoselenadiazole-Doped Metal-Organic Frameworks
- PMID: 36558041
- PMCID: PMC9781904
- DOI: 10.3390/molecules27248908
Photodynamic Inactivation of Bacteria and Biofilms with Benzoselenadiazole-Doped Metal-Organic Frameworks
Abstract
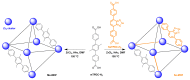
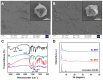
Bacterial biofilms are difficult to treat due to their resistance to traditional antibiotics. Although photodynamic therapy (PDT) has made significant progress in biomedical applications, most photosensitizers have poor water solubility and can thus aggregate in hydrophilic environments, leading to the quenching of photosensitizing activity in PDT. Herein, a benzoselenadiazole-containing ligand was designed and synthesized to construct the zirconium (IV)-based benzoselenadiazole-doped metal-organic framework (Se-MOF). Characterizations revealed that Se-MOF is a type of UiO-68 topological framework with regular crystallinity and high porosity. Compared to the MOF without benzoselenadiazole, Se-MOF exhibited a higher 1O2 generation efficacy and could effectively kill Staphylococcus aureus bacteria under visible-light irradiation. Importantly, in vitro biofilm experiments confirmed that Se-MOF could efficiently inhibit the formation of bacteria biofilms upon visible-light exposure. This study provides a promising strategy for developing MOF-based PDT agents, facilitating their transformation into clinical photodynamic antibacterial applications.
Keywords: antimicrobial agents; bacteria; biofilms; irradiation; metal-organic frameworks.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ćirić A.D., Petrović J.D., Glamočlija J.M., Smiljković M.S., Nikolić M.M., Stojković D.S., Soković M.D. Natural Products as Biofilm Formation Antagonists and Negulators of Quorum Sensing Functions: A Comprehensive Review Update and Future Trends. S. Afr. J. Bot. 2019;120:65–80. doi: 10.1016/j.sajb.2018.09.010. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

