Self-Supplying Guide RNA-Mediated CRISPR/Cas12a Fluorescence System for Sensitive Detection of T4 PNKP
- PMID: 36558152
- PMCID: PMC9782049
- DOI: 10.3390/molecules27249019
Self-Supplying Guide RNA-Mediated CRISPR/Cas12a Fluorescence System for Sensitive Detection of T4 PNKP
Abstract
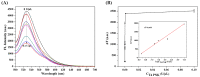
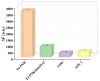
Sensitive detection methods for T4 polynucleotide kinase/phosphatase (T4 PNKPP) are urgently required to obtain information on malignancy and thereby to provide better guidance in PNKP-related diagnostics and drug screening. Although the CRISPR/Cas12a system shows great promise in DNA-based signal amplification protocols, its guide RNAs with small molecular weight often suffer nuclease degradation during storage and utilization, resulting in reduced activation efficiency. Herein, we proposed a self-supplying guide RNA-mediated CRISPR/Cas12a system for the sensitive detection of T4 PNKP in cancer cells, in which multiple copies of guide RNA were generated by in situ transcription. In this assay, T4 PNKP was chosen as a model, and a dsDNA probe with T7 promoter region and the transcription region of guide RNA were involved. Under the action of T4 PNKP, the 5'-hydroxyl group of the dsDNA probe was converted to a phosphate group, which can be recognized and digested by Lambda Exo, resulting in dsDNA hydrolysis. The transcription template was destroyed, which resulted in the failure to generate guide RNA by the transcription pathway. Therefore, the CRISPR/Cas12a system could not be activated to effectively cleavage the F-Q-reporter, and the fluorescence signal was turned off. In the absence of T4 PNKP, the 5'-hydroxyl group of the substrate DNA cannot be digested by Lambda Exo. The intact dsDNA acts as the transcription template to generate a large amount of guide RNA. Finally, the formed Cas12a/gRNA complex triggered the reverse cleavage of Cas12a on the F-Q-reporter, resulting in a "turn-on" fluorescence signal. This strategy displayed sharp sensitivity of T4 PNKP with the limit of detection (LOD) down to 0.0017 mU/mL, which was mainly due to the multiple regulation effect of transcription amplification. In our system, the dsDNA simultaneously serves as the T4 PNKP substrate, transcription template, and Lambda Exo substrate, avoiding the need for multiple probe designs and saving costs. By integrating the target recognition, Lambda Exo activity, and trans-cleavage activity of Cas12a, CRISPR/Cas12a catalyzed the cleavage of fluorescent-labeled short-stranded DNA probes and enabled synergetic signal amplification for sensitive T4 PNKP detection. Furthermore, the T4 PNKP in cancer cells has been evaluated as a powerful tool for biomedical research and clinical diagnosis, proving a good practical application capacity.
Keywords: CRISPR/Cas12a; T4 PNKP; transcription.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
CRISPR/Cas12a-based dual amplified biosensing system for sensitive and rapid detection of polynucleotide kinase/phosphatase.Biosens Bioelectron. 2020 Nov 15;168:112556. doi: 10.1016/j.bios.2020.112556. Epub 2020 Aug 27. Biosens Bioelectron. 2020. PMID: 32890931
-
Probing CRISPR-Cas12a Nuclease Activity Using Double-Stranded DNA-Templated Fluorescent Substrates.Biochemistry. 2020 Apr 21;59(15):1474-1481. doi: 10.1021/acs.biochem.0c00140. Epub 2020 Apr 7. Biochemistry. 2020. PMID: 32233423 Free PMC article.
-
Thioflavin T as a fluorescence probe for label-free detection of T4 polynucleotide kinase/phosphatase and its inhibitors.Mol Cell Probes. 2015 Dec;29(6):500-502. doi: 10.1016/j.mcp.2015.11.002. Epub 2015 Nov 11. Mol Cell Probes. 2015. PMID: 26577032
-
Application of CRISPR/Cas12a in the rapid detection of pathogens.Clin Chim Acta. 2023 Aug 1;548:117520. doi: 10.1016/j.cca.2023.117520. Epub 2023 Aug 16. Clin Chim Acta. 2023. PMID: 37595863 Review.
-
Making the cut(s): how Cas12a cleaves target and non-target DNA.Biochem Soc Trans. 2019 Oct 31;47(5):1499-1510. doi: 10.1042/BST20190564. Biochem Soc Trans. 2019. PMID: 31671185 Review.
References
-
- Mao J., Chen X., Xu H., Xu X. DNAzyme-driven DNAWalker Biosensor for Amplified Electrochemical Detection of T4 polyNucleotide Kinase Activity and Inhibition. J. Electroanal. Chem. 2020;874:114470. doi: 10.1016/j.jelechem.2020.114470. - DOI
-
- Luo R., Zhou H., Dang W., Long Y., Tong C., Xie Q., Daniyal M., Liu B., Wang W. A DNAzyme-rGO Coupled Fluorescence Assay for T4 PNKP Activity in Vitro and Intracellular Imaging. Sens. Actuat B-Chem. 2020;310:127884. doi: 10.1016/j.snb.2020.127884. - DOI
-
- Lin L., Shi D., Li Q., Wang G.F., Zhang X.J. Detection of T4 Polynucleotide Kinase Based on a MnO2 Nanosheet-3,3′,5,5′-Tetramethylbenzidine (TMB) Colorimetric System. Anal. Methods. 2016;8:4119–4126. doi: 10.1039/C6AY00269B. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

