Potential Antioxidant Activity of Apigenin in the Obviating Stress-Mediated Depressive Symptoms of Experimental Mice
- PMID: 36558188
- PMCID: PMC9787100
- DOI: 10.3390/molecules27249055
Potential Antioxidant Activity of Apigenin in the Obviating Stress-Mediated Depressive Symptoms of Experimental Mice
Abstract
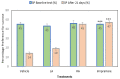
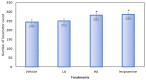
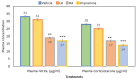
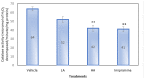
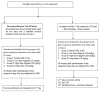
This study aimed to examine the antidepressant properties of apigenin in an experimental mouse model of chronic mild stress (CMS). Three weeks following CMS, albino mice of either sex were tested for their antidepressant effects using the tail suspension test (TST) and the sucrose preference test. The percentage preference for sucrose solution and the amount of time spent immobile in the TST were calculated. The brain malondialdehyde (MDA) levels, catalase activity, and reduced glutathione levels were checked to determine the antioxidant potential of treatments. When compared to the control, animals treated with apigenin during the CMS periods showed significantly shorter TST immobility times. Apigenin administration raised the percentage preference for sucrose solution in a dose-dependent manner, which put it on par with the widely used antidepressant imipramine. Animals treated with apigenin displayed a significantly (p ˂ 0.05) greater spontaneous locomotor count (281) when compared to the vehicle-treated group (245). Apigenin was also highly effective in significantly (p ˂ 0.01) lowering plasma corticosterone levels (17 vs. 28 µg/mL) and nitrite (19 vs. 33 µg/mL) produced by CMS in comparison to the control group. During CMS, a high dose (50 mg/kg) of apigenin was given, which greatly increased the reduced glutathione level while significantly decreasing the brain's MDA and catalase activity when compared to the control group. As a result, we infer that high doses of apigenin may have potential antidepressant effects in animal models via various mechanisms.
Keywords: antidepressant activity; antioxidant potential; apigenin; chronic mild stress; sucrose preference test; tail suspension test.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Antidepressant Effect of Crocin in Mice with Chronic Mild Stress.Molecules. 2022 Aug 25;27(17):5462. doi: 10.3390/molecules27175462. Molecules. 2022. PMID: 36080230 Free PMC article.
-
Antidepressant-like effects of Sanyuansan in the mouse forced swim test, tail suspension test, and chronic mild stress model.Kaohsiung J Med Sci. 2015 Dec;31(12):605-12. doi: 10.1016/j.kjms.2015.10.009. Epub 2015 Nov 27. Kaohsiung J Med Sci. 2015. PMID: 26709221 Free PMC article.
-
Antidepressant-like activity of plumbagin in unstressed and stressed mice.Pharmacol Rep. 2015 Oct;67(5):1024-32. doi: 10.1016/j.pharep.2015.03.001. Epub 2015 Mar 19. Pharmacol Rep. 2015. PMID: 26398399
-
Apigenin reverses depression-like behavior induced by chronic corticosterone treatment in mice.Eur J Pharmacol. 2016 Mar 5;774:50-4. doi: 10.1016/j.ejphar.2016.01.015. Epub 2016 Jan 27. Eur J Pharmacol. 2016. PMID: 26826594
-
Antidepressant-like behavioral and neurochemical effects of the citrus-associated chemical apigenin.Life Sci. 2008 Mar 26;82(13-14):741-51. doi: 10.1016/j.lfs.2008.01.007. Epub 2008 Jan 30. Life Sci. 2008. PMID: 18308340
Cited by
-
Therapeutic potential of natural products in inflammation: underlying molecular mechanisms, clinical outcomes, technological advances, and future perspectives.Inflammopharmacology. 2023 Dec;31(6):2857-2883. doi: 10.1007/s10787-023-01366-y. Epub 2023 Nov 11. Inflammopharmacology. 2023. PMID: 37950803 Review.
-
Antidepressant activity of flavones from traditional Chinese medicine: a meta-analysis.Pharm Biol. 2025 Dec;63(1):156-169. doi: 10.1080/13880209.2025.2467374. Epub 2025 Feb 25. Pharm Biol. 2025. PMID: 39996320 Free PMC article.
-
Apigenin targets fetuin-A to ameliorate obesity-induced insulin resistance.Int J Biol Sci. 2024 Feb 11;20(5):1563-1577. doi: 10.7150/ijbs.91695. eCollection 2024. Int J Biol Sci. 2024. PMID: 38481798 Free PMC article.
-
The Beneficial Role of Apigenin against Cognitive and Neurobehavioural Dysfunction: A Systematic Review of Preclinical Investigations.Biomedicines. 2024 Jan 13;12(1):178. doi: 10.3390/biomedicines12010178. Biomedicines. 2024. PMID: 38255283 Free PMC article. Review.
-
Neuroprotective effects of Apigenin on prenatal Valproic acid-induced autism spectrum disorder in rats.IBRO Neurosci Rep. 2024 Oct 30;17:493-502. doi: 10.1016/j.ibneur.2024.10.003. eCollection 2024 Dec. IBRO Neurosci Rep. 2024. PMID: 39720795 Free PMC article.
References
-
- Leonard B., Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concimatants play a role in the pathophysiology of unipolar depression. Neurosci. Biobehav. Rev. 2012;36:764–785. doi: 10.1016/j.neubiorev.2011.12.005. - DOI - PubMed
-
- Tanabe A., Nomura S., Rinsho N. Pathophysiology of depression. Nihon Rinsho. 2007;65:1585–1590. - PubMed
-
- Maes M., Galecki P., Chang Y.S., Berk M. A review on the oxidative and nitrosative stress (O & NS) pathways in major depression and their possible contribution to the (neuro) degenerative process in that illness. Prog. Neuro-Psychophar. Macol. Biol. Psychiatr. 2011;35:676–692. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

