Impact of Fecal Microbiota Transplantation on Gut Bacterial Bile Acid Metabolism in Humans
- PMID: 36558359
- PMCID: PMC9785599
- DOI: 10.3390/nu14245200
Impact of Fecal Microbiota Transplantation on Gut Bacterial Bile Acid Metabolism in Humans
Abstract
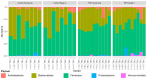
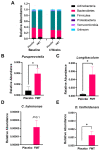
Fecal microbiota transplantation (FMT) is a promising therapeutic modality for the treatment and prevention of metabolic disease. We previously conducted a double-blind, randomized, placebo-controlled pilot trial of FMT in obese metabolically healthy patients in which we found that FMT enhanced gut bacterial bile acid metabolism and delayed the development of impaired glucose tolerance relative to the placebo control group. Therefore, we conducted a secondary analysis of fecal samples collected from these patients to assess the potential gut microbial species contributing to the effect of FMT to improve metabolic health and increase gut bacterial bile acid metabolism. Fecal samples collected at baseline and after 4 weeks of FMT or placebo treatment underwent shotgun metagenomic analysis. Ultra-high-performance liquid chromatography-mass spectrometry was used to profile fecal bile acids. FMT-enriched bacteria that have been implicated in gut bile acid metabolism included Desulfovibrio fairfieldensis and Clostridium hylemonae. To identify candidate bacteria involved in gut microbial bile acid metabolism, we assessed correlations between bacterial species abundance and bile acid profile, with a focus on bile acid products of gut bacterial metabolism. Bacteroides ovatus and Phocaeicola dorei were positively correlated with unconjugated bile acids. Bifidobacterium adolescentis, Collinsella aerofaciens, and Faecalibacterium prausnitzii were positively correlated with secondary bile acids. Together, these data identify several candidate bacteria that may contribute to the metabolic benefits of FMT and gut bacterial bile acid metabolism that requires further functional validation.
Keywords: bile acids; bile salt hydrolase (BSH); fecal microbiome transplant (FMT); gut microbiota; metagenomics.
Conflict of interest statement
J.-M.B., C.L., Z.M., J.R.M., K.A.C., A.R. and B.P.C. have no relevant conflict of interest to declare. J.R.A. consults for and has research support from Finch Therapeutics Group, Janssen, Pfizer, Abbvie, Iterative Sopes, Seres Therapeutics, Ferring, Merck, Bristol Myer Squibb and has research support from Pfizer and Merck. T.D. has research support from AMPEL BioSolutions. B.H.M. has received consultancy fees from Finch Therapeutics Group and Ferring Pharmaceuticals. C.C.T. consults for Apollo Endosurgery, Boston Scientific, Medtronic, Enterasense Ltd., EnVision Endoscopy, Fractyl, Fujifilm, GI Dynamics, GI Windows, Lumendi, Olympus, USGI Medical, Xenter, Endoquest Robotics. He has received Research Support from Apollo Endosurgery, Boston Scientific, ERBE, Fujifilm, GI Dynamics, Lumedi Olympus, USGI Medical. He serves on Advisory Boards for Fractyl, Fujifilm, USGI Medical, Xenter and Endoquest Robotics. He is a founder, board member and receives ownership interest from Enterasense Ltd., EnVision Endoscopy and GI Windows, He is on a speakers bureau for Boston Scientific, Fujifilm and Olympus. He receives royalty payments from GI Windows, EndoSim and Enterasense Ltd.
Figures