Heat-Killed Bifidobacterium bifidum B1628 May Alleviate Dextran Sulfate Sodium-Induced Colitis in Mice, and the Anti-Inflammatory Effect Is Associated with Gut Microbiota Modulation
- PMID: 36558391
- PMCID: PMC9784753
- DOI: 10.3390/nu14245233
Heat-Killed Bifidobacterium bifidum B1628 May Alleviate Dextran Sulfate Sodium-Induced Colitis in Mice, and the Anti-Inflammatory Effect Is Associated with Gut Microbiota Modulation
Abstract
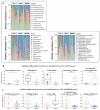
Inflammatory bowel disease (IBD) is a chronic inflammatory disease associated with gut dysbiosis. This study aimed to investigate the effects of heat-killed Bifidobacterium bifidum B1628 (HB1628) in dextran sulfate sodium (DSS)-induced colitis in mice. The following three mouse groups were included (n = eight per group): NC (normal control), DSS (colitis), and HB1628 (colitis and postbiotic). The mice in the DSS group showed significant weight loss and histological damage, developed bloody diarrhea, scored high in the disease activity index (DAI), and exhibited increases in pro-inflammatory cytokines (interleukin [IL]-1β, IL-6, and tumor necrosis factor [TNF]-α) and decreases in an anti-inflammatory cytokine (IL-13) in the serum. These changes were accompanied by gut microbiota modulation in colitis mice (decreases in Rikenellaceae and Eubacterium; increases in Peptostreptococcaceae, Bacteroides vulgatus, and Parasutterella excrementihominis). The HB1628 group had lower DAIs, histology scores, and serum levels of pro-inflammatory cytokines (IL-1β and TNF-α), but higher levels of an anti-inflammatory cytokine (IL-13), compared with the DSS group, suggesting a less severe inflammatory state after the HB1628 intervention. Additionally, HB1628 improved DSS-induced gut dysbiosis, which is evidenced by increases in intestinal beneficial bacteria, such as Lactobacillus, and decreases in known unfavorable taxa in IBD, e.g., Porphyromonadaceae, Subdoligranulum, Lachnospiraceae bacterium 3_1_46FAA, and Alistipes indistinctus. Functional metagenomics revealed three significantly enriched metabolic pathways in the HB1628 group (namely, the aerobic respiration I [cytochrome c] pathway and the superpathways of L-phenylalanine biosynthesis and L-tryptophan biosynthesis, respectively). In conclusion, our results showed that HB1628 effectively improved the inflammation state and tissue damage in DSS-induced colitis mice, and the symptom relief effect was accompanied by obvious gut microbiota remodulation.
Keywords: Bifidobacterium bifidum; anti-inflammation; gut microbiota; heat-killed; inflammatory bowel disease; postbiotics.
Conflict of interest statement
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Figures


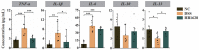


References
-
- Turner D., Ricciuto A., Lewis A., D’Amico F., Dhaliwal J., Griffiths A.M., Bettenworth D., Sandborn W.J., Sands B.E., Reinisch W., et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology. 2021;160:1570–1583. doi: 10.1053/j.gastro.2020.12.031. - DOI - PubMed
-
- Teofani A., Marafini I., Laudisi F., Pietrucci D., Salvatori S., Unida V., Biocca S., Monteleone G., Desideri A. Intestinal taxa abundance and diversity in inflammatory bowel disease patients: An analysis including covariates and confounders. Nutrients. 2022;14:260. doi: 10.3390/nu14020260. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

