Interplay between Gut Microbiota and NLRP3 Inflammasome in Intracerebral Hemorrhage
- PMID: 36558410
- PMCID: PMC9788242
- DOI: 10.3390/nu14245251
Interplay between Gut Microbiota and NLRP3 Inflammasome in Intracerebral Hemorrhage
Abstract
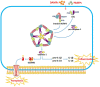
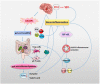
The pathophysiological process of intracerebral hemorrhage (ICH) is very complex, involving various mechanisms such as apoptosis, oxidative stress and inflammation. As one of the key factors, the inflammatory response is responsible for the pathological process of acute brain injury and is associated with the prognosis of patients. Abnormal or dysregulated inflammatory responses after ICH can aggravate cell damage in the injured brain tissue. The NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome is a multiprotein complex distributed in the cytosol, which can be triggered by multiple signals. The NLRP3 inflammasome is activated after ICH, thus promoting neuroinflammation and aggravating brain edema. In addition, there is evidence that the gut microbiota is crucial in the activation of the NLRP3 inflammasome. The gut microbiota plays a key role in a variety of CNS disorders. Changes in the diversity and species of the gut microbiota affect neuroinflammation through the activation of the NLRP3 inflammasome and the release of inflammatory cytokines. In turn, the gut microbiota composition can be influenced by the activation of the NLRP3 inflammasome. Thereby, the regulation of the microbe-gut-brain axis via the NLRP3 inflammasome may serve as a novel idea for protecting against secondary brain injury (SBI) in ICH patients. Here, we review the recent evidence on the functions of the NLRP3 inflammasome and the gut microbiota in ICH, as well as their interactions, during the pathological process of ICH.
Keywords: NLRP3 inflammasome; gut microbiota; intracerebral hemorrhage; secondary brain injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Targeting NLRP3 inflammasome modulates gut microbiota, attenuates corticospinal tract injury and ameliorates neurobehavioral deficits after intracerebral hemorrhage in mice.Biomed Pharmacother. 2022 May;149:112797. doi: 10.1016/j.biopha.2022.112797. Epub 2022 Mar 10. Biomed Pharmacother. 2022. PMID: 35279596
-
Secondary White Matter Injury Mediated by Neuroinflammation after Intracerebral Hemorrhage and Promising Therapeutic Strategies of Targeting the NLRP3 Inflammasome.Curr Neuropharmacol. 2023;21(3):669-686. doi: 10.2174/1570159X20666220830115018. Curr Neuropharmacol. 2023. PMID: 36043798 Free PMC article. Review.
-
iTRAQ-Based Quantitative Proteomics Indicated Nrf2/OPTN-Mediated Mitophagy Inhibits NLRP3 Inflammasome Activation after Intracerebral Hemorrhage.Oxid Med Cell Longev. 2021 Feb 9;2021:6630281. doi: 10.1155/2021/6630281. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33628368 Free PMC article.
-
Neuroinflammation Mediated by NLRP3 Inflammasome After Intracerebral Hemorrhage and Potential Therapeutic Targets.Mol Neurobiol. 2020 Dec;57(12):5130-5149. doi: 10.1007/s12035-020-02082-2. Epub 2020 Aug 27. Mol Neurobiol. 2020. PMID: 32856203 Review.
-
Ghrelin attenuates secondary brain injury following intracerebral hemorrhage by inhibiting NLRP3 inflammasome activation and promoting Nrf2/ARE signaling pathway in mice.Int Immunopharmacol. 2020 Feb;79:106180. doi: 10.1016/j.intimp.2019.106180. Epub 2020 Jan 8. Int Immunopharmacol. 2020. PMID: 31926478
Cited by
-
The role of gut microorganisms and metabolites in intracerebral hemorrhagic stroke: a comprehensive review.Front Neurosci. 2024 Feb 21;18:1346184. doi: 10.3389/fnins.2024.1346184. eCollection 2024. Front Neurosci. 2024. PMID: 38449739 Free PMC article. Review.
-
Brain-Gut-Microbiota Axis in Amyotrophic Lateral Sclerosis: A Historical Overview and Future Directions.Aging Dis. 2024 Feb 1;15(1):74-95. doi: 10.14336/AD.2023.0524. Aging Dis. 2024. PMID: 37307822 Free PMC article. Review.
-
Microglial Mechanisms and Therapeutic Potential in Brain Injury Post-Intracerebral Hemorrhage.J Inflamm Res. 2025 Feb 26;18:2955-2973. doi: 10.2147/JIR.S498809. eCollection 2025. J Inflamm Res. 2025. PMID: 40026311 Free PMC article. Review.
-
The causal effects between gut microbiota and hemorrhagic stroke: a bidirectional two-sample Mendelian randomization study.Front Microbiol. 2023 Dec 22;14:1290909. doi: 10.3389/fmicb.2023.1290909. eCollection 2023. Front Microbiol. 2023. PMID: 38188561 Free PMC article.
-
Crosstalk between ubiquitin ligases and ncRNAs drives cardiovascular disease progression.Front Immunol. 2024 Mar 7;15:1335519. doi: 10.3389/fimmu.2024.1335519. eCollection 2024. Front Immunol. 2024. PMID: 38515760 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

