Potential Benefits of Lycopene Consumption: Rationale for Using It as an Adjuvant Treatment for Malaria Patients and in Several Diseases
- PMID: 36558462
- PMCID: PMC9787606
- DOI: 10.3390/nu14245303
Potential Benefits of Lycopene Consumption: Rationale for Using It as an Adjuvant Treatment for Malaria Patients and in Several Diseases
Erratum in
-
Correction: Varela et al. Potential Benefits of Lycopene Consumption: Rationale for Using It as an Adjuvant Treatment for Malaria Patients and in Several Diseases. Nutrients 2022, 14, 5303.Nutrients. 2024 Oct 16;16(20):3503. doi: 10.3390/nu16203503. Nutrients. 2024. PMID: 39458570 Free PMC article.
Abstract
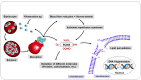
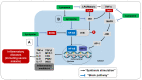
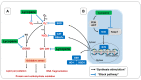
Malaria is a disease that affects thousands of people around the world every year. Its pathogenesis is associated with the production of reactive oxygen and nitrogen species (RONS) and lower levels of micronutrients and antioxidants. Patients under drug treatment have high levels of oxidative stress biomarkers in the body tissues, which limits the use of these drugs. Therefore, several studies have suggested that RONS inhibition may represent an adjuvant therapeutic strategy in the treatment of these patients by increasing the antioxidant capacity of the host. In this sense, supplementation with antioxidant compounds such as zinc, selenium, and vitamins A, C, and E has been suggested as part of the treatment. Among dietary antioxidants, lycopene is the most powerful antioxidant among the main carotenoids. This review aimed to describe the main mechanisms inducing oxidative stress during malaria, highlighting the production of RONS as a defense mechanism against the infection induced by the ischemia-reperfusion syndrome, the metabolism of the parasite, and the metabolism of antimalarial drugs. Furthermore, the effects of lycopene on several diseases in which oxidative stress is implicated as a cause are outlined, providing information about its mechanism of action, and providing an evidence-based justification for its supplementation in malaria.
Keywords: adjuvant treatment; antioxidants; carotenoids; lycopene; malaria; oxidative stress; supplementation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Lycopene: A Potent Antioxidant for the Amelioration of Type II Diabetes Mellitus.Molecules. 2022 Apr 4;27(7):2335. doi: 10.3390/molecules27072335. Molecules. 2022. PMID: 35408734 Free PMC article. Review.
-
[Carotenoids as natural antioxidants].Postepy Hig Med Dosw (Online). 2015 Apr 7;69:418-28. doi: 10.5604/17322693.1148335. Postepy Hig Med Dosw (Online). 2015. PMID: 25897101 Review. Polish.
-
Lycopene and Its Antioxidant Role in the Prevention of Cardiovascular Diseases-A Critical Review.Crit Rev Food Sci Nutr. 2016 Aug 17;56(11):1868-79. doi: 10.1080/10408398.2013.801827. Crit Rev Food Sci Nutr. 2016. PMID: 25675359 Review.
-
Lycopene and beta-carotene protect against oxidative damage in HT29 cells at low concentrations but rapidly lose this capacity at higher doses.Free Radic Res. 1999 Feb;30(2):141-51. doi: 10.1080/10715769900300151. Free Radic Res. 1999. PMID: 10193582
-
Oxidative and antioxidant status in plasma of runners: effect of oral supplementation with natural antioxidants.J Med Food. 2009 Feb;12(1):145-50. doi: 10.1089/jmf.2008.0074. J Med Food. 2009. PMID: 19298208 Clinical Trial.
Cited by
-
Correction: Varela et al. Potential Benefits of Lycopene Consumption: Rationale for Using It as an Adjuvant Treatment for Malaria Patients and in Several Diseases. Nutrients 2022, 14, 5303.Nutrients. 2024 Oct 16;16(20):3503. doi: 10.3390/nu16203503. Nutrients. 2024. PMID: 39458570 Free PMC article.
-
The Association between Malaria and β-Carotene Levels: A Systematic Review and Meta-Analysis.Antioxidants (Basel). 2023 Aug 29;12(9):1687. doi: 10.3390/antiox12091687. Antioxidants (Basel). 2023. PMID: 37759990 Free PMC article. Review.
-
The Role of Oxidative Stress and Natural Products in Maintaining Human Health.Nutrients. 2024 Apr 25;16(9):1268. doi: 10.3390/nu16091268. Nutrients. 2024. PMID: 38732515 Free PMC article.
-
Lycopene: A Potent Antioxidant with Multiple Health Benefits.J Nutr Metab. 2024 Jun 8;2024:6252426. doi: 10.1155/2024/6252426. eCollection 2024. J Nutr Metab. 2024. PMID: 38883868 Free PMC article. Review.
-
Oxidative Stress in Parasitic Diseases-Reactive Oxygen Species as Mediators of Interactions between the Host and the Parasites.Antioxidants (Basel). 2023 Dec 23;13(1):38. doi: 10.3390/antiox13010038. Antioxidants (Basel). 2023. PMID: 38247462 Free PMC article. Review.
References
-
- WHO . Word Malaria Report 2021. World Health Organization; Geneva, Switzerland: 2021.
-
- van Wolfswinkel M.E., Langenberg M.C.C., Wammes L.J., Sauerwein R.W., Koelewijn R., Hermsen C.C., van Hellemond J.J., van Genderen P.J. Changes in Total and Differential Leukocyte Counts during the Clinically Silent Liver Phase in a Controlled Human Malaria Infection in Malaria-Naïve Dutch Volunteers. Malar. J. 2017;16:457. doi: 10.1186/s12936-017-2108-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

