Modeling Obesity-Associated Ovarian Dysfunction in Drosophila
- PMID: 36558524
- PMCID: PMC9783805
- DOI: 10.3390/nu14245365
Modeling Obesity-Associated Ovarian Dysfunction in Drosophila
Abstract
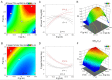
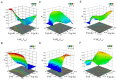
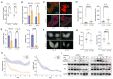
We perform quantitative studies to investigate the effect of high-calorie diet on Drosophila oogenesis. We use the central composite design (CCD) method to obtain quadratic regression models of body fat and fertility as a function of the concentrations of protein and sucrose, two major macronutrients in Drosophila diet, and treatment duration. Our results reveal complex interactions between sucrose and protein in impacting body fat and fertility when they are considered as an integrated physiological response. We verify the utility of our quantitative modeling approach by experimentally confirming the physiological responses-including increased body fat, reduced fertility, and ovarian insulin insensitivity-expected of a treatment condition identified by our modeling method. Under this treatment condition, we uncover a Drosophila oogenesis phenotype that exhibits an accumulation of immature oocytes and a halt in the production of mature oocytes, a phenotype that bears resemblance to key aspects of the human condition of polycystic ovary syndrome (PCOS). Our analysis of the dynamic progression of different aspects of diet-induced pathophysiology also suggests an order of the onset timing for obesity, ovarian dysfunction, and insulin resistance. Thus, our study documents the utility of quantitative modeling approaches toward understanding the biology of Drosophila female reproduction, in relation to diet-induced obesity and type II diabetes, serving as a potential disease model for human ovarian dysfunction.
Keywords: Drosophila; animal model; central composite design; insulin resistance; obesity; ovarian dysfunction.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Celik E., Olson E., Titi E.S. Spectral Filtering of Interpolant Observables for a Discrete-in-Time Downscaling Data Assimilation Algorithm. SIAM J. Appl. Dyn. Syst. 2019;18:1118–1142. doi: 10.1137/18M1218480. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

