Filoviruses: Innate Immunity, Inflammatory Cell Death, and Cytokines
- PMID: 36558734
- PMCID: PMC9785368
- DOI: 10.3390/pathogens11121400
Filoviruses: Innate Immunity, Inflammatory Cell Death, and Cytokines
Abstract
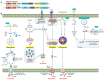
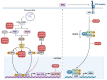
Filoviruses are a group of single-stranded negative sense RNA viruses. The most well-known filoviruses that affect humans are ebolaviruses and marburgviruses. During infection, they can cause life-threatening symptoms such as inflammation, tissue damage, and hemorrhagic fever, with case fatality rates as high as 90%. The innate immune system is the first line of defense against pathogenic insults such as filoviruses. Pattern recognition receptors (PRRs), including toll-like receptors, retinoic acid-inducible gene-I-like receptors, C-type lectin receptors, AIM2-like receptors, and NOD-like receptors, detect pathogens and activate downstream signaling to induce the production of proinflammatory cytokines and interferons, alert the surrounding cells to the threat, and clear infected and damaged cells through innate immune cell death. However, filoviruses can modulate the host inflammatory response and innate immune cell death, causing an aberrant immune reaction. Here, we discuss how the innate immune system senses invading filoviruses and how these deadly pathogens interfere with the immune response. Furthermore, we highlight the experimental difficulties of studying filoviruses as well as the current state of filovirus-targeting therapeutics.
Keywords: ALRs; CLRs; NLRs; PANoptosis; PANoptosome; RIG-I; RLRs; RNA virus; TLRs; apoptosis; caspase; cell death; ebolavirus; filovirus; host–pathogen interactions; inflammasome; inflammation; innate immunity; interferon; marburgvirus; necroptosis; pattern recognition receptors; pyroptosis.
Conflict of interest statement
T.-D.K. is a consultant for Pfizer.
Figures
Similar articles
-
Mechanisms and pathways of innate immune activation and regulation in health and cancer.Hum Vaccin Immunother. 2014;10(11):3270-85. doi: 10.4161/21645515.2014.979640. Hum Vaccin Immunother. 2014. PMID: 25625930 Free PMC article. Review.
-
Innate and intrinsic antiviral immunity in skin.J Dermatol Sci. 2014 Sep;75(3):159-66. doi: 10.1016/j.jdermsci.2014.05.004. Epub 2014 Jun 2. J Dermatol Sci. 2014. PMID: 24928148 Review.
-
Pattern-recognition receptors in endometriosis: A narrative review.Front Immunol. 2023 Mar 23;14:1161606. doi: 10.3389/fimmu.2023.1161606. eCollection 2023. Front Immunol. 2023. PMID: 37033937 Free PMC article. Review.
-
Immune regulator IRF1 contributes to ZBP1-, AIM2-, RIPK1-, and NLRP12-PANoptosome activation and inflammatory cell death (PANoptosis).J Biol Chem. 2023 Sep;299(9):105141. doi: 10.1016/j.jbc.2023.105141. Epub 2023 Aug 7. J Biol Chem. 2023. PMID: 37557956 Free PMC article.
-
Innate Immune Sensing of Viruses and Its Consequences for the Central Nervous System.Viruses. 2021 Jan 23;13(2):170. doi: 10.3390/v13020170. Viruses. 2021. PMID: 33498715 Free PMC article. Review.
Cited by
-
Novel antiviral approaches for Marburg: a promising therapeutics in the pipeline.Front Microbiol. 2024 Apr 25;15:1387628. doi: 10.3389/fmicb.2024.1387628. eCollection 2024. Front Microbiol. 2024. PMID: 38725678 Free PMC article. Review.
-
Peripheral immune responses to filoviruses in a reservoir versus spillover hosts reveal transcriptional correlates of disease.Front Immunol. 2024 Jan 8;14:1306501. doi: 10.3389/fimmu.2023.1306501. eCollection 2023. Front Immunol. 2024. PMID: 38259437 Free PMC article.
-
Therapeutic advances in Marburg virus disease: from experimental treatments to vaccine development.Ann Med Surg (Lond). 2025 Mar 28;87(5):2784-2799. doi: 10.1097/MS9.0000000000003213. eCollection 2025 May. Ann Med Surg (Lond). 2025. PMID: 40337393 Free PMC article. Review.
-
Ebola Virus Infection Induces HCAR2 Expression Leading to Cell Death.J Infect Dis. 2023 Nov 13;228(Suppl 7):S508-S513. doi: 10.1093/infdis/jiad344. J Infect Dis. 2023. PMID: 37578011 Free PMC article.
-
The Interplay Between Viral Infection and Cell Death: A Ping-Pong Effect.Adv Virol. 2025 Feb 3;2025:5750575. doi: 10.1155/av/5750575. eCollection 2025. Adv Virol. 2025. PMID: 39959654 Free PMC article. Review.
References
-
- Slenczka W. Filovirus research: How it began. Curr. Top. Microbiol. Immunol. 2017;411:3–21. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

