The Characterization of Cardiac Explants Reveals Unique Fibrosis Patterns and a Predominance of CD8+ T Cell Subpopulations in Patients with Chronic Chagas Cardiomyopathy
- PMID: 36558736
- PMCID: PMC9788058
- DOI: 10.3390/pathogens11121402
The Characterization of Cardiac Explants Reveals Unique Fibrosis Patterns and a Predominance of CD8+ T Cell Subpopulations in Patients with Chronic Chagas Cardiomyopathy
Abstract
Aim: The present study aimed to characterize the histopathological findings and the phenotype of inflammatory cells in the myocardial tissue of patients with end-stage heart failure (ESHF) secondary to CCC in comparison with ESHF secondary to non-Chagas cardiomyopathies (NCC).
Methods: A total of 32 explanted hearts were collected from transplanted patients between 2014 and 2017. Of these, 21 were classified as CCC and 11 as other NCC. A macroscopic analysis followed by a microscopic analysis were performed. Finally, the phenotypes of the inflammatory infiltrates were characterized using flow cytometry.
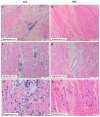
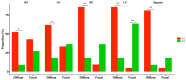
Results: Microscopic analysis revealed more extensive fibrotic involvement in patients with CCC, with more frequent foci of fibrosis, collagen deposits, and degeneration of myocardial fibers, in addition to identifying foci of inflammatory infiltrate of greater magnitude. Finally, cell phenotyping identified more memory T cells, mainly CD8+CD45RO+ T cells, and fewer transitioning T cells (CD45RA+/CD45RO+) in patients with CCC compared with the NCC group.
Conclusions: CCC represents a unique form of myocardial involvement characterized by abundant inflammatory infiltrates, severe interstitial fibrosis, extensive collagen deposits, and marked cardiomyocyte degeneration. The structural myocardial changes observed in late-stage Chagas cardiomyopathy appear to be closely related to the presence of cardiac fibrosis and the colocalization of collagen fibers and inflammatory cells, a finding that serves as a basis for the generation of new hypotheses aimed at better understanding the role of inflammation and fibrogenesis in the progression of CCC. Finally, the predominance of memory T cells in CCC compared with NCC hearts highlights the critical role of the parasite-specific lymphocytic response in the course of the infection.
Keywords: chagas disease; chronic chagas cardiomyopathy; histopathology; immunophenotyping.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




Similar articles
-
T-Cell Subpopulations Exhibit Distinct Recruitment Potential, Immunoregulatory Profile and Functional Characteristics in Chagas versus Idiopathic Dilated Cardiomyopathies.Front Cardiovasc Med. 2022 Feb 2;9:787423. doi: 10.3389/fcvm.2022.787423. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35187122 Free PMC article.
-
Metabolomic Profiling of End-Stage Heart Failure Secondary to Chronic Chagas Cardiomyopathy.Int J Mol Sci. 2022 Sep 9;23(18):10456. doi: 10.3390/ijms231810456. Int J Mol Sci. 2022. PMID: 36142367 Free PMC article.
-
Chagas cardiomyopathy is associated with a high susceptibility to T. cruzi infection in monocyte-derived macrophages and a predominance of CD4+CD45RO+ T-cells with immunoregulatory patterns.Acta Trop. 2023 Jan;237:106749. doi: 10.1016/j.actatropica.2022.106749. Epub 2022 Nov 10. Acta Trop. 2023. PMID: 36370753
-
Myocardial fibrosis in chagas disease and molecules related to fibrosis.Parasite Immunol. 2019 Oct;41(10):e12663. doi: 10.1111/pim.12663. Epub 2019 Aug 11. Parasite Immunol. 2019. PMID: 31309590 Review.
-
Myocardial gene and protein expression profiles after autoimmune injury in Chagas' disease cardiomyopathy.Autoimmun Rev. 2011 Jan;10(3):163-5. doi: 10.1016/j.autrev.2010.09.019. Epub 2010 Sep 29. Autoimmun Rev. 2011. PMID: 20883825 Review.
Cited by
-
Correlation of blood-based immune molecules with cardiac gene expression profiles reveals insights into Chagas cardiomyopathy pathogenesis.Front Immunol. 2024 Feb 8;15:1338582. doi: 10.3389/fimmu.2024.1338582. eCollection 2024. Front Immunol. 2024. PMID: 38390336 Free PMC article.
-
Immunologic changes are detectable in the peripheral blood transcriptome of clinically asymptomatic Chagas cardiomyopathy patients.bioRxiv [Preprint]. 2023 Oct 5:2023.10.03.560680. doi: 10.1101/2023.10.03.560680. bioRxiv. 2023. Update in: Lancet Reg Health Am. 2025 Apr 17;45:101090. doi: 10.1016/j.lana.2025.101090. PMID: 37873108 Free PMC article. Updated. Preprint.
-
Immunomodulation and immunopharmacology in heart failure.Nat Rev Cardiol. 2024 Feb;21(2):119-149. doi: 10.1038/s41569-023-00919-6. Epub 2023 Sep 14. Nat Rev Cardiol. 2024. PMID: 37709934 Review.
-
Immunologic changes in the peripheral blood transcriptome of individuals with early-stage chronic Chagas cardiomyopathy: a cross-sectional study.Lancet Reg Health Am. 2025 Apr 17;45:101090. doi: 10.1016/j.lana.2025.101090. eCollection 2025 May. Lancet Reg Health Am. 2025. PMID: 40290486 Free PMC article.
-
Mechanisms behind the high mortality rate in chronic Chagas cardiomyopathy: Unmasking a three-headed monster.Eur J Heart Fail. 2024 Dec;26(12):2502-2514. doi: 10.1002/ejhf.3460. Epub 2024 Sep 26. Eur J Heart Fail. 2024. PMID: 39327798 Free PMC article. Review.
References
-
- Nunes M.C.P., Beaton A., Acquatella H., Bern C., Bolger A.F., Echeverría L.E., Dutra W.O., Gascon J., Morillo C.A., Oliveira-Filho J., et al. Chagas Cardiomyopathy: An Update of Current Clinical Knowledge and Management: A Scientific Statement From the American Heart Association. Circulation. 2018;138:e169–e209. doi: 10.1161/CIR.0000000000000599. - DOI - PubMed
-
- World Health Organization . Weekly Epidemiological Record. Volume 90. World Health Organization; Geneva, Switzerland: 2015. [(accessed on 28 March 2022)]. Chagas Disease in Latin America: An Epidemiological Update Based on 2010 Estimates. Available online: https://www.who.int/wer/2015/wer9006.pdf?ua=1.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

