Nipah and Hendra Viruses: Deadly Zoonotic Paramyxoviruses with the Potential to Cause the Next Pandemic
- PMID: 36558753
- PMCID: PMC9784551
- DOI: 10.3390/pathogens11121419
Nipah and Hendra Viruses: Deadly Zoonotic Paramyxoviruses with the Potential to Cause the Next Pandemic
Abstract
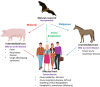
Nipah and Hendra viruses are deadly zoonotic paramyxoviruses with a case fatality rate of upto 75%. The viruses belong to the genus henipavirus in the family Paramyxoviridae, a family of negative-sense single-stranded RNA viruses. The natural reservoirs of NiV and HeV are bats (flying foxes) in which the virus infection is asymptomatic. The intermediate hosts for NiV and HeV are swine and equine, respectively. In humans, NiV infections result in severe and often fatal respiratory and neurological manifestations. The Nipah virus was first identified in Malaysia and Singapore following an outbreak of encephalitis in pig farmers and subsequent outbreaks have been reported in Bangladesh and India almost every year. Due to its extreme pathogenicity, pandemic potential, and lack of established antiviral therapeutics and vaccines, research on henipaviruses is highly warranted so as to develop antivirals or vaccines that could aid in the prevention and control of future outbreaks.
Keywords: BSL-4; Cedar virus; Hendra virus; Nipah virus; vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Henipavirus zoonosis: outbreaks, animal hosts and potential new emergence.Front Microbiol. 2023 Jul 17;14:1167085. doi: 10.3389/fmicb.2023.1167085. eCollection 2023. Front Microbiol. 2023. PMID: 37529329 Free PMC article. Review.
-
Hendra virus and Nipah virus animal vaccines.Vaccine. 2016 Jun 24;34(30):3525-34. doi: 10.1016/j.vaccine.2016.03.075. Epub 2016 May 4. Vaccine. 2016. PMID: 27154393 Free PMC article.
-
Discovery and Genomic Characterization of a Novel Henipavirus, Angavokely Virus, from Fruit Bats in Madagascar.J Virol. 2022 Sep 28;96(18):e0092122. doi: 10.1128/jvi.00921-22. Epub 2022 Aug 30. J Virol. 2022. PMID: 36040175 Free PMC article.
-
Hendra and Nipah viruses: pathogenesis and therapeutics.Curr Mol Med. 2005 Dec;5(8):805-16. doi: 10.2174/156652405774962308. Curr Mol Med. 2005. PMID: 16375714 Review.
-
Third Helical Domain of the Nipah Virus Fusion Glycoprotein Modulates both Early and Late Steps in the Membrane Fusion Cascade.J Virol. 2020 Sep 15;94(19):e00644-20. doi: 10.1128/JVI.00644-20. Print 2020 Sep 15. J Virol. 2020. PMID: 32669342 Free PMC article.
Cited by
-
Henipavirus zoonosis: outbreaks, animal hosts and potential new emergence.Front Microbiol. 2023 Jul 17;14:1167085. doi: 10.3389/fmicb.2023.1167085. eCollection 2023. Front Microbiol. 2023. PMID: 37529329 Free PMC article. Review.
-
Enzyme-Linked Immunosorbent Assay Using Henipavirus-Receptor EphrinB2 and Monoclonal Antibodies for Detecting Nipah and Hendra Viruses.Viruses. 2024 May 16;16(5):794. doi: 10.3390/v16050794. Viruses. 2024. PMID: 38793674 Free PMC article.
-
Emerging and reemerging infectious diseases: global trends and new strategies for their prevention and control.Signal Transduct Target Ther. 2024 Sep 11;9(1):223. doi: 10.1038/s41392-024-01917-x. Signal Transduct Target Ther. 2024. PMID: 39256346 Free PMC article. Review.
-
Universal paramyxovirus vaccine design by stabilizing regions involved in structural transformation of the fusion protein.Nat Commun. 2024 May 31;15(1):4629. doi: 10.1038/s41467-024-48059-w. Nat Commun. 2024. PMID: 38821950 Free PMC article.
-
Is the emergence of the zoonotic Langya virus amidst COVID-19 and monkeypox a cause for concern?Future Virol. 2023 Jan;18(1):5-7. doi: 10.2217/fvl-2022-0175. Epub 2023 Jan 27. Future Virol. 2023. PMID: 36864889 Free PMC article. No abstract available.
References
-
- WHO Nipah Virus. [(accessed on 6 August 2022)];2018 Available online: https://www.who.int/news-room/fact-sheets/detail/nipah-virus.
-
- Epstein J.H., Anthony S.J., Islam A., Kilpatrick A.M., Khan S.A., Balkey M.D., Ross N., Smith I., Zambrana-Torrelio C., Tao Y., et al. Nipah virus dynamics in bats and implications for spillover to humans. Proc. Natl. Acad. Sci. USA. 2020;117:29190–29201. doi: 10.1073/pnas.2000429117. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

