Differential Expression of Immune Genes in the Rhipicephalus microplus Gut in Response to Theileria equi Infection
- PMID: 36558812
- PMCID: PMC9782190
- DOI: 10.3390/pathogens11121478
Differential Expression of Immune Genes in the Rhipicephalus microplus Gut in Response to Theileria equi Infection
Abstract
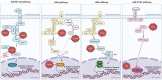
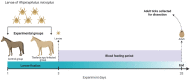
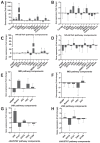
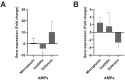
Rhipicephalus microplus is the only tick species known to serve as a biological vector of Theileria equi for horses and other equids in Brazil. The protozoan T. equi is one of the causal agents of equine piroplasmosis, a major threat in horse breeding systems. Vector competence is closely linked to the pathogens' ability to evade tick defense mechanisms. However, knowledge of tick immune response against infections by hemoparasites of the Theileria genus is scarce. In the present study, the expression of genes involved in immune signaling pathways of R. microplus adults' guts when challenged with a high or low parasitic load of T. equi was evaluated. This research demonstrates divergences in the immune gene expression pattern linked to T. equi infection in R. microplus since the Toll, IMD, and JNK signaling pathways were transcriptionally repressed in the guts of adult ticks infected with T. equi. Moreover, the results showed that different infectious doses of T. equi induce differential gene expression of key components of immune signaling cascades in R. microplus gut, suggesting a link between the intensity of infection and the activation of tick immunity response. The present study adds knowledge to elucidate the gut immune signaling response of R. microplus to T. equi infection. In addition, the generated data can serve as a basis for further investigations to develop strategies for controlling and preventing equine piroplasmosis.
Keywords: horses; immunity; parasite–vector relationship; signaling pathways; tick.
Conflict of interest statement
The authors declare no conflict of interest. The funding sponsors had no role in the study’s design, experimental procedures, analyses, interpretation of data, writing of the manuscript, or decision to publish the results.
Figures
Similar articles
-
Comparison of Seroprevalence and Identification of Risk Factors for Theileria equi in Horses From Vector-Free and Infested Areas in Southern Brazil.J Equine Vet Sci. 2023 Jul;126:104241. doi: 10.1016/j.jevs.2023.104241. Epub 2023 Feb 10. J Equine Vet Sci. 2023. PMID: 36773853
-
Dynamics of Theileria equi Infection in Rhipicephalus (Boophilus) microplus during the Parasitic Phase in a Chronically Infected Horse.Pathogens. 2022 Apr 29;11(5):525. doi: 10.3390/pathogens11050525. Pathogens. 2022. PMID: 35631046 Free PMC article.
-
Molecular and serological detection of Theileria equi and Babesia caballi infection in horses and ixodid ticks in Iran.Ticks Tick Borne Dis. 2014 Apr;5(3):239-44. doi: 10.1016/j.ttbdis.2013.11.008. Epub 2014 Feb 17. Ticks Tick Borne Dis. 2014. PMID: 24556274
-
Review of equine piroplasmosis.J Vet Intern Med. 2013 Nov-Dec;27(6):1334-46. doi: 10.1111/jvim.12168. Epub 2013 Aug 28. J Vet Intern Med. 2013. PMID: 24033559 Review.
-
Vector ecology of equine piroplasmosis.Annu Rev Entomol. 2015 Jan 7;60:561-80. doi: 10.1146/annurev-ento-010814-021110. Annu Rev Entomol. 2015. PMID: 25564746 Review.
Cited by
-
Investigation of genes expression of the JAK/STAT signalling pathway and AMPs in the presence of Borrelia spirochetes in Ixodes ricinus.Sci Rep. 2025 Jan 22;15(1):2869. doi: 10.1038/s41598-025-87506-6. Sci Rep. 2025. PMID: 39843584 Free PMC article.
References
-
- Friedhoff K.T., Tenter A.M., Müller I. Haemoparasites of Equines: Impact on International Trade of Horses. Rev. Sci. Et Tech. 1990;9:1187–1194. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

