Cryopreservation of Plasmodium Sporozoites
- PMID: 36558821
- PMCID: PMC9784981
- DOI: 10.3390/pathogens11121487
Cryopreservation of Plasmodium Sporozoites
Abstract
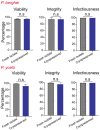
Malaria is a deadly disease caused by the parasite, Plasmodium, and impacts the lives of millions of people around the world. Following inoculation into mammalian hosts by infected mosquitoes, the sporozoite stage of Plasmodium undergoes obligate development in the liver before infecting erythrocytes and causing clinical malaria. The most promising vaccine candidates for malaria rely on the use of attenuated live sporozoites to induce protective immune responses. The scope of widespread testing or clinical use of such vaccines is limited by the absence of efficient, reliable, or transparent strategies for the long-term preservation of live sporozoites. Here we outline a method to cryopreserve the sporozoites of various human and murine Plasmodium species. We found that the structural integrity, viability, and in vivo or in vitro infectiousness were conserved in the recovered cryopreserved sporozoites. Cryopreservation using our approach also retained the transgenic properties of sporozoites and immunization with cryopreserved radiation attenuated sporozoites (RAS) elicited strong immune responses. Our work offers a reliable protocol for the long-term storage and recovery of human and murine Plasmodium sporozoites and lays the groundwork for the widespread use of live sporozoites for research and clinical applications.
Keywords: Plasmodium; RAS vaccination; cryopreservation; freezing; malaria; sporozoite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- WHO . World Malaria Report 2021. World Health Organization; Geneva, Switzerland: 2021.
-
- RTS,S Clinical Trials Partnership Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45. doi: 10.1016/S0140-6736(15)60721-8. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

