Effect of Dexamethasone on the Expression of the α2,3 and α2,6 Sialic Acids in Epithelial Cell Lines
- PMID: 36558852
- PMCID: PMC9788320
- DOI: 10.3390/pathogens11121518
Effect of Dexamethasone on the Expression of the α2,3 and α2,6 Sialic Acids in Epithelial Cell Lines
Abstract
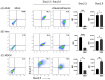
N-acetylneuraminic acid linked to galactose by α2,6 and α2,3 linkages (Siaα2,6 and Siaα2,3) is expressed on glycoconjugates of animal tissues, where it performs multiple biological functions. In addition, these types of sialic acid residues are the main targets for the binding and entry of influenza viruses. Here we used fluorochrome-conjugated Sambuccus nigra, Maackia amurensis, and peanut lectins for the simultaneous detection of Siaα2,3 and Siaα2,6 and galactosyl residues by two-color flow cytometry on A549 cells, a human pneumocyte cell line used for in vitro studies of the infection by influenza viruses, as well as on Vero and MDCK cell lines. The dexamethasone (DEX) glucocorticoid (GC), a widely used anti-inflammatory compound, completely abrogated the expression of Siaα2,3 in A549 cells and decreased its expression in Vero and MDCK cells; in contrast, the expression of Siaα2,6 was increased in the three cell lines. These observations indicate that DEX can be used for the study of the mechanism of sialylation of cell membrane molecules. Importantly, DEX may change the tropism of avian and human/pig influenza viruses and other infectious agents to animal and human epithelial cells.
Keywords: A549; MDCK; Vero; dexamethasone; influenza receptors; sialic acid; sialylation; zoonosis.
Conflict of interest statement
The authors declare that there are no conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

