IL-10-Secreting CD8+ T Cells Specific for Human Cytomegalovirus (HCMV): Generation, Maintenance and Phenotype
- PMID: 36558866
- PMCID: PMC9781655
- DOI: 10.3390/pathogens11121530
IL-10-Secreting CD8+ T Cells Specific for Human Cytomegalovirus (HCMV): Generation, Maintenance and Phenotype
Abstract
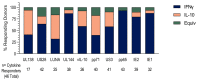
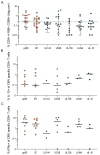
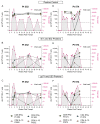
HCMV-specific CD8+ T-cells are potent anti-viral effector cells in HCMV infected individuals, but evidence from other viral infections suggests that CD8+ T-cells can also produce the immunomodulatory cytokine IL-10. In this work we show that there are HCMV-specific IL-10 CD8+ T-cell responses in a cohort of individuals aged 23-76 years of age, predominantly directed against the HCMV proteins known to be expressed during latent infections as well as towards the proteins US3 and pp71. The analysis of HCMV-specific responses established during primary infection has shown that the IL-10 responses to US3 and pp71 HCMV proteins are detectable in the first weeks post infection, but not the responses to latency-associated proteins, and this IL-10 response is produced by both CD8+ and CD4+ T-cells. Phenotyping studies of HCMV-specific IL-10+ CD8+ T-cells show that these are CD45RA+ effector memory cells and co-express CD28 and CD57, however, the expression of the inhibitory receptor PD-1 varied from 90% to 30% between donors. In this study we have described for the first time the HCMV-specific IL-10 CD8+ T-cell responses and have demonstrated their broad specificity and the potential immune modulatory role of the immune response to HCMV latent carriage and periodic reactivation.
Keywords: CD8+ T cells; IL-10 secretion; T regulatory cells; human cytomegalovirus (HCMV); immunomodulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

