rCsHscB Derived from Clonorchis sinensis: A Carcinogenic Liver Fluke Ameliorates LPS-Induced Acute Hepatic Injury by Repression of Inflammation
- PMID: 36558882
- PMCID: PMC9782140
- DOI: 10.3390/pathogens11121548
rCsHscB Derived from Clonorchis sinensis: A Carcinogenic Liver Fluke Ameliorates LPS-Induced Acute Hepatic Injury by Repression of Inflammation
Abstract
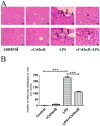
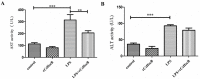
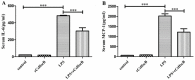
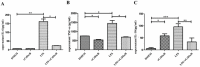
Sepsis-associated acute liver injury caused by spillovers of bacteria and endotoxins (lipopolysaccharide, LPS) into the liver remains a public health issue due to the lack of specific therapeutic approaches. Previous studies showed that the recombinant protein HscB (rCsHscB) of Clonorchis sinensis, a carcinogenic liver fluke, had an anti-inflammatory effect and could alleviate inflammatory diseases such as enteritis; however, whether it can prevent sepsis-associated acute liver injury induced by LPS is still unknown. In our current study, the therapeutic effects and the potential mechanisms of rCsHscB on LPS-induced acute liver injury were investigated both in vivo and in vitro. The data showed that rCsHscB prevented LPS-induced liver damage, as demonstrated by histopathological observation and hepatic damage markers (the activities of serum ALT and AST) in a murine model of sepsis-associated acute liver injury. rCsHscB also significantly reversed the high levels of serum IL-6 and MCP-1 induced by LPS. In addition, rCsHscB attenuated the production of LPS-induced proinflammatory cytokines, including IL-6 and TNF-α, in a macrophage cell line-RAW264.7, through possible mediation by the MAPK signaling pathway in vitro. In conclusion, the present study demonstrates that rCsHscB derived from a fluke C. sinensis protects against sepsis-associated acute liver injury induced by LPS, which may be attributed to the inhibition of the MAPK signaling pathway. Our present study provides a potential therapeutic strategy for sepsis-associated acute liver injury.
Keywords: Clonorchis sinensis; LPS; MAPK; rCsHscB; sepsis-associated liver injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
CsHscB Derived from a Liver Fluke Clonorchis sinensis Ameliorates Cholestatic Hepatic Fibrosis in a Mouse Model of Sclerosing Cholangitis.Curr Mol Med. 2024;24(4):505-515. doi: 10.2174/1566524023666230418111949. Curr Mol Med. 2024. PMID: 37076961
-
Recombinant CsHscB of carcinogenic liver fluke Clonorchis sinensis induces IL-10 production by binding with TLR2.PLoS Negl Trop Dis. 2020 Oct 12;14(10):e0008643. doi: 10.1371/journal.pntd.0008643. eCollection 2020 Oct. PLoS Negl Trop Dis. 2020. PMID: 33044969 Free PMC article.
-
Clonorchis sinensis adult-derived proteins elicit Th2 immune responses by regulating dendritic cells via mannose receptor.PLoS Negl Trop Dis. 2018 Mar 5;12(3):e0006251. doi: 10.1371/journal.pntd.0006251. eCollection 2018 Mar. PLoS Negl Trop Dis. 2018. PMID: 29505573 Free PMC article.
-
Preventive effects of interleukin-6 in lipopolysaccharide/d-galactosamine induced acute liver injury via regulating inflammatory response in hepatic macrophages.Int Immunopharmacol. 2017 Oct;51:99-106. doi: 10.1016/j.intimp.2017.08.009. Epub 2017 Aug 17. Int Immunopharmacol. 2017. PMID: 28822324
-
The Brief Case: Incidental finding of a liver fluke following resection of hepatocellular carcinoma.J Clin Microbiol. 2025 Jan 31;63(1):e0130224. doi: 10.1128/jcm.01302-24. Epub 2025 Jan 31. J Clin Microbiol. 2025. PMID: 39887204 Free PMC article. Review. No abstract available.
References
Grants and funding
- 201810313033Y/Training Programs of innovation and Entrepreneurship for College Students in Jiangsu Province
- 82172297/National Natural Science Foundation of China
- 22KJA310007/Natural Science Foundation of Jiangsu Higher Education Institutions of China
- BK20211346/Natural Science Foundation of Jiangsu Province of China
LinkOut - more resources
Full Text Sources
Miscellaneous

