A Comparison of Doxycycline and Amoxicillin Containing Quadruple Eradication Therapy for Treating Helicobacter pylori-Infected Duodenal Ulcers: A Multicenter, Opened, Randomized Controlled Trial in China
- PMID: 36558883
- PMCID: PMC9783029
- DOI: 10.3390/pathogens11121549
A Comparison of Doxycycline and Amoxicillin Containing Quadruple Eradication Therapy for Treating Helicobacter pylori-Infected Duodenal Ulcers: A Multicenter, Opened, Randomized Controlled Trial in China
Abstract
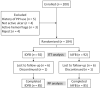
Background: Increased antibiotic resistance is one of the major factors contributing to the failure of H. pylori eradication. This study aimed to compare the efficacy and safety of doxycycline and amoxicillin, both critical components for bismuth-based quadruple therapy, for the first-line treatment of H. pylori-infected duodenal ulcers. Methods: An open, randomized case-controlled, multicenter trial was conducted in seven hospitals in China. A total of 184 eligible participants were divided into an IDFB (ilaprazole 5 mg, doxycycline 100 mg, furazolidone 100 mg, and bismuth 220 mg bid) or IAFB (ilaprazole 5 mg, amoxicillin 1000 mg, furazolidone 100 mg, and bismuth 220 mg bid) group for 14 days. Both groups were administrated with ilaprazole 5 mg qd for another 14 days. The main outcome was an H. pylori eradication rate; secondary outcomes were ulcer healing, relief of symptoms, and incidence of adverse effects. Results: The H. pylori eradication rates were 85.9% (95% CI 78.6−93.9) in the IDFB vs. 84.8% (95% CI 77.3−92.3) in the IAFB group in ITT analysis (p > 0.05), and 92.9% (95% CI 87.4−98.5) vs. and 91.8% (95% CI 85.8−97.7) in PP analysis (p > 0.05). The overall ulcer healing rates of IDFB and IAFB were 79.1% and 84.7% (p > 0.05), both effective in relieving symptoms. Only nine participants had adverse reactions in this trial (4/92 in IDFB and 5/92 in IAFB). Conclusion: A bismuth quadruple regimen containing doxycycline or amoxicillin could be an effective and safe treatment for H. pylori eradication, while doxycycline replacement is an alternative for participants with penicillin allergy.
Keywords: Helicobacter pylori; antibiotic resistance; doxycycline; eradication; penicillin allergy; safety.
Conflict of interest statement
All authors declare no conflict of interest.
Figures
Similar articles
-
Comparison of the Dual Therapy of Ilaprazole-Amoxicillin and the Bismuth Quadruple Therapy of Ilaprazole-Amoxicillin-Furazolidone-Bismuth Glycyrrhizinate for Eradication of Helicobacter pylori.Front Pharmacol. 2022 Apr 27;13:771876. doi: 10.3389/fphar.2022.771876. eCollection 2022. Front Pharmacol. 2022. PMID: 35571120 Free PMC article.
-
Comparative study of allicin-containing quadruple therapy vs. bismuth-containing quadruple therapy for the treatment of Helicobacter pylori infection: a prospective randomized study.Eur J Gastroenterol Hepatol. 2021 Feb 1;32(2):194-200. doi: 10.1097/MEG.0000000000001896. Eur J Gastroenterol Hepatol. 2021. PMID: 32804837 Clinical Trial.
-
[Ilaprazole based bismuth-containing quadruple regimen for the first-line treatment of Helicobacter pylori infection: a multicenter, randomized, controlled clinical study].Zhonghua Yi Xue Za Zhi. 2012 Aug 14;92(30):2108-12. Zhonghua Yi Xue Za Zhi. 2012. PMID: 23158273 Clinical Trial. Chinese.
-
Ilaprazole-amoxicillin dual therapy at high dose as a first-line treatment for helicobacter pylori infection in Hainan: a single-center, open-label, noninferiority, randomized controlled trial.BMC Gastroenterol. 2023 Jul 24;23(1):249. doi: 10.1186/s12876-023-02890-5. BMC Gastroenterol. 2023. PMID: 37488516 Free PMC article. Clinical Trial.
-
Second-line rescue treatment of Helicobacter pylori infection: Where are we now?World J Gastroenterol. 2018 Oct 28;24(40):4548-4553. doi: 10.3748/wjg.v24.i40.4548. World J Gastroenterol. 2018. PMID: 30386104 Free PMC article. Review.
Cited by
-
Newer, Older, and Alternative Agents for the Eradication of Helicobacter pylori Infection: A Narrative Review.Antibiotics (Basel). 2023 May 23;12(6):946. doi: 10.3390/antibiotics12060946. Antibiotics (Basel). 2023. PMID: 37370265 Free PMC article. Review.
-
Clinical Effectiveness of Penicillin-Free Therapies in First-Line and Rescue Treatments for Helicobacter pylori: A Systematic Review.Antibiotics (Basel). 2025 May 8;14(5):476. doi: 10.3390/antibiotics14050476. Antibiotics (Basel). 2025. PMID: 40426542 Free PMC article. Review.
-
Molecular Mechanisms of Antibiotic Resistance and Novel Treatment Strategies for Helicobacter pylori Infections.Trop Med Infect Dis. 2023 Mar 11;8(3):163. doi: 10.3390/tropicalmed8030163. Trop Med Infect Dis. 2023. PMID: 36977164 Free PMC article. Review.
References
-
- Ford A.C., Forman D., Hunt R.H., Yuan Y., Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: Systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174. doi: 10.1136/bmj.g3174. - DOI - PMC - PubMed
-
- El-Serag H.B., Kao J.Y., Kanwal F., Gilger M., LoVecchio F., Moss S.F., Crowe S.E., Elfant A., Haas T., Hapke R.J., et al. Houston Consensus Conference on Testing for Helicobacter pylori Infection in the United States. Clin. Gastroenterol. Hepatol. 2018;16:992–1002.e6. doi: 10.1016/j.cgh.2018.03.013. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous