The MEK Inhibitor Trametinib Improves Outcomes following Subarachnoid Haemorrhage in Female Rats
- PMID: 36558896
- PMCID: PMC9785726
- DOI: 10.3390/ph15121446
The MEK Inhibitor Trametinib Improves Outcomes following Subarachnoid Haemorrhage in Female Rats
Abstract
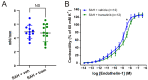
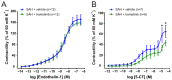
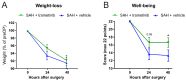
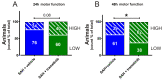
Aneurysmal subarachnoid haemorrhage (SAH) is a haemorrhagic stroke that causes approximately 5% of all stroke incidents. We have been working on a treatment strategy that targets changes in cerebrovascular contractile receptors, by blocking the MEK/ERK1/2 signalling pathway. Recently, a positive effect of trametinib was found in male rats, but investigations of both sexes in pre-clinical studies are an important necessity. In the current study, a SAH was induced in female rats, by autologous blood-injection into the pre-chiasmatic cistern. This produces a dramatic, transient increase in intracranial pressure (ICP) and an acute and prolonged decrease in cerebral blood flow. Rats were then treated with either vehicle or three doses of 0.5 mg/kg trametinib (specific MEK/ERK1/2 inhibitor) intraperitoneally at 3, 9, and 24 h after the SAH. The outcome was assessed by a panel of tests, including intracranial pressure (ICP), sensorimotor tests, a neurological outcome score, and myography. We observed a significant difference in arterial contractility and a reduction in subacute increases in ICP when the rats were treated with trametinib. The sensory motor and neurological outcomes in trametinib-treated rats were significantly improved, suggesting that the improved outcome in females is similar to that of males treated with trametinib.
Keywords: ET-1; SAH; cerebral artery; rat; subarachnoid haemorrhage; trametinib.
Conflict of interest statement
Nothing to declare by any of the authors.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

