Analyzing the Difference in the Length of Stay (LOS) in Moderate to Severe COVID-19 Patients Receiving Hydroxychloroquine or Favipiravir
- PMID: 36558907
- PMCID: PMC9785070
- DOI: 10.3390/ph15121456
Analyzing the Difference in the Length of Stay (LOS) in Moderate to Severe COVID-19 Patients Receiving Hydroxychloroquine or Favipiravir
Abstract
Background: The coronavirus 2019 (COVID-19) disease, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus led to a global pandemic. HCQ and FPV were used early in the pandemic as a treatment modality for COVID-19. Various studies evaluated the HCQ and FPV effectiveness, based on the mortality endpoint and showed conflicting results. We hypothesize that analyzing the difference in the LOS as a significant endpoint would be of a major interest, especially for healthcare providers, to prevent a lengthy hospitalization and disease progression. Methods: This is a retrospective observational study, conducted via a medical chart review of COVD-19 patients who were admitted between April 2020 and March 2021 with a moderate to severe illness. The LOS endpoint was tested using the paired Wilcoxon signed-rank (WSR) model. Prior to using the WSR model, the balance between the HCQ and FPV groups, the propensity score matching, the LOS distribution, and the normality assumptions were tested. Two sensitivity statistical analyses were conducted to confirm the results (stratified log-rank test and U Welch test after transforming the LOS by the squared root values). Results: A total of 200 patients were included for the analysis: 83 patients in the HCQ group and 117 patients in the FPV group. Thirty-seven patients were matched in each group. The LOS data was positively skewed and violated the normality (Shapiro−Wilk p < 0.001) and had an unequal variance (Levene’s test, p = 0.019). The WSR test showed no statistical significance in the LOS endpoint, with a median of −0.75 days (95% confidence interval: −4.0 to 2.5, p = 0.629), in favor of the HCQ group (four days), in comparison to seven days of the FPV group. The WSR findings were further confirmed with the stratified log rank test (p = 740) and the U Welch test (p = 391). Conclusions: The study concluded that the HCQ and FPV treatments have a comparable effectiveness in terms of the LOS in the moderate to severe COVID-19 patients. This study highlights the importance of analyzing the LOS as a relevant endpoint, in order to prevent the costs of a lengthy hospitalization and disease progression. The current study also emphasizes the importance of applying the appropriate statistical testing when dealing with two-sample paired data and analyzing non-parametric data such as the LOS.
Keywords: COVID-19; SARS-CoV-2; effectiveness; favipiravir; hydroxychloroquine; length of stay; statistical analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
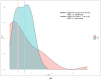

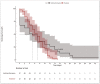
References
-
- WHO . Coronavirus Disease (COVID-19) Situation Report. World Health Organization; Geneva, Switzerland: 2022.
-
- COVID-19 Treatment Guidelines Panel Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. [(accessed on 4 April 2022)]; Available online: https://www.covid19treatmentguidelines.nih.gov/
-
- Alotaibi M., Ali A., Bakhshwin D., Alatawi Y., Alotaibi S., Alhifany A., Alharthi B., Alharthi N., Alyazidi A., Alharthi Y., et al. Effectiveness and Safety of Favipiravir Compared to Hydroxychloroquine for Management of COVID-19: A Retrospective Study. Int. J. Gen. Med. 2021;14:5597–5606. doi: 10.2147/IJGM.S329881. - DOI - PMC - PubMed
-
- Costanzo M., De Giglio M.A.R., Roviello G.N. SARS-CoV-2: Recent Reports on Antiviral Therapies Based on Lopinavir/Ritonavir, Darunavir/Umifenovir, Hydroxychloroquine, Remdesivir, Favipiravir and other Drugs for the Treatment of the New Coronavirus. Curr. Med. Chem. 2020;27:4536–4541. doi: 10.2174/0929867327666200416131117. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

