Update on COVID-19 Therapy in Pediatric Age
- PMID: 36558963
- PMCID: PMC9783267
- DOI: 10.3390/ph15121512
Update on COVID-19 Therapy in Pediatric Age
Abstract
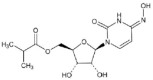
With the extension of the COVID-19 pandemic, the large use of COVID-19 vaccines among adults and the emergence of SARS-CoV-2 variants means that the epidemiology of COVID-19 in pediatrics, particularly among younger children, has substantially changed. The prevalence of pediatric COVID-19 significantly increased, several severe cases among children were reported, and long-COVID in pediatric age was frequently observed. The main aim of this paper is to discuss which types of treatment are presently available for pediatric patients with COVID-19, which of them are authorized for the first years of life, and which are the most important limitations of COVID-19 therapy in pediatric age. Four different antivirals, remdesivir (RVD), the combination nirmatrelvir plus ritonavir (Paxlovid), molnupiravir (MPV), and the monoclonal antibody bebtelovimab (BEB), are presently approved or authorized for emergency use for COVID-19 treatment by most of the national health authorities, although with limitations according to the clinical relevance of disease and patient's characteristics. Analyses in the literature show that MPV cannot be used in pediatric age for the risk of adverse events regarding bone growth. The other antivirals can be used, at least in older children, and RDV can be used in all children except in neonates. However, careful research on pharmacokinetic and clinical data specifically collected in neonates and children are urgently needed for the appropriate management of pediatric COVID-19.
Keywords: COVID-19; antiviral therapy; bebtelovimab; molnupiravir; nirmatrelvir/ritonavir; remdesivir.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Garazzino S., Montagnani C., Donà D., Meini A., Felici E., Vergine G., Bernardi S., Giacchero R., Lo Vecchio A., Marchisio P., et al. Multicentre Italian study of SARS-CoV-2 infection in children and adolescents, preliminary data as at 10 April 2020. Euro Surveill. 2020;25:2000600. doi: 10.2807/1560-7917.ES.2020.25.18.2000600. - DOI - PMC - PubMed
-
- American Academy of Pediatrics and the Children’s Hospital Association Children and COVID-19: State Data Report. [(accessed on 6 October 2022)]. Available online: https://downloads.aap.org/AAP/PDF/AAP%20and%20CHA%20-%20Children%20and%2....
-
- Food and Drug Administration FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Children 5 through 11 Years of Age. [(accessed on 10 October 2022)]; Available online: https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfize....
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous