Gastrointestinal Permeation Enhancers for the Development of Oral Peptide Pharmaceuticals
- PMID: 36559036
- PMCID: PMC9781085
- DOI: 10.3390/ph15121585
Gastrointestinal Permeation Enhancers for the Development of Oral Peptide Pharmaceuticals
Abstract
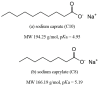
Recently, two oral-administered peptide pharmaceuticals, semaglutide and octreotide, have been developed and are considered as a breakthrough in peptide and protein drug delivery system development. In 2019, the Food and Drug Administration (FDA) approved an oral dosage form of semaglutide developed by Novo Nordisk (Rybelsus®) for the treatment of type 2 diabetes. Subsequently, the octreotide capsule (Mycapssa®), developed through Chiasma's Transient Permeation Enhancer (TPE) technology, also received FDA approval in 2020 for the treatment of acromegaly. These two oral peptide products have been a significant success; however, a major obstacle to their oral delivery remains the poor permeability of peptides through the intestinal epithelium. Therefore, gastrointestinal permeation enhancers are of great relevance for the development of subsequent oral peptide products. Sodium salcaprozate (SNAC) and sodium caprylate (C8) have been used as gastrointestinal permeation enhancers for semaglutide and octreotide, respectively. Herein, we briefly review two approved products, Rybelsus® and Mycapssa®, and discuss the permeation properties of SNAC and medium chain fatty acids, sodium caprate (C10) and C8, focusing on Eligen technology using SNAC, TPE technology using C8, and gastrointestinal permeation enhancement technology (GIPET) using C10.
Keywords: medium chain fatty acids; oral delivery; peptides; permeation enhancers; sodium salcaprozate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

